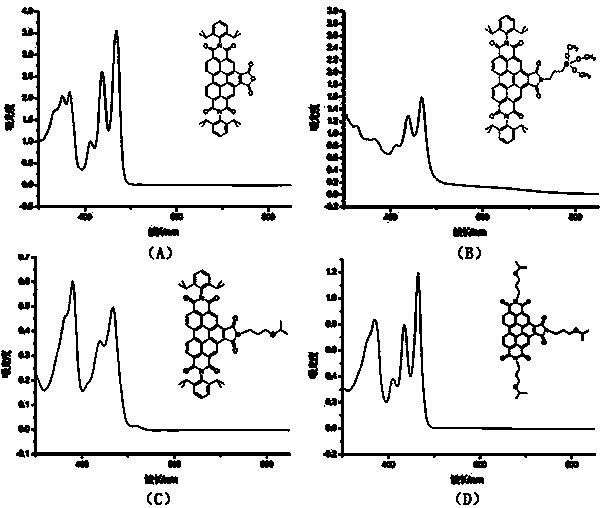
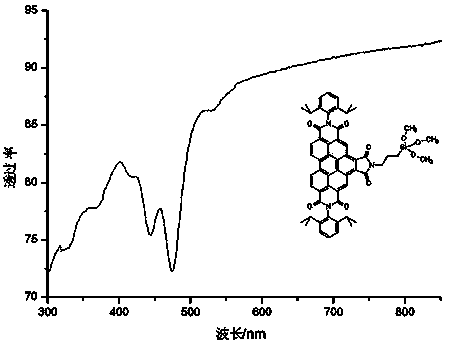
Benzoperylene imide derivatives, and preparation method and application thereof
A technology of benzoperyleneimide and its derivatives, which is applied in the field of benzoperyleneimide derivatives and its preparation, can solve the problems that the value of blue light radiation absorption has not been developed and applied, and the synthesis route has not been comprehensively summarized.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
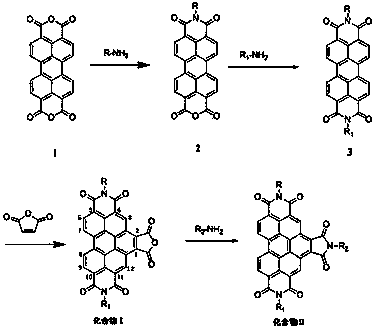
[0032] Example 1 N,N-bis(2,6-diisopropylphenyl)-1,2,4,5,10,11-benzoperylenehexacarboxylic acid-1,2-anhydride-4,5,10,11 -Synthesis of imides
[0033] 1) Add 1g (2.55mmol) 3,4,9,10-perylenetetracarboxylic dianhydride (compound 1), 2mL (10.6mmol) 2,6-diisopropylaniline and 7g imidazole into a single-necked flask, mix well Then react at 130°C for 24 h under the protection of argon, and after cooling to room temperature, pour the reaction solution into a mixed solution of 50 mL ethanol and 60 mL 2M hydrochloric acid, filter with suction, wash the obtained solid with water twice, and then wash it with saturated NaHCO 3 The solution was washed twice, then washed with water until neutral, dried in a vacuum oven at 85°C and purified by silica gel column chromatography. 1 g of the obtained solid was added to 2 mL (10.6 mmol) of 2,6-diisopropylaniline and 7 g of Repeat the steps after imidazole to obtain 1.2 g dark red solid (compound 2), yield 66%;
[0034] 2) After preheating 20g of
Embodiment 2
[0035] Example 2 N,N-bis(2,6 diisopropylphenyl)-N-(3-trimethylsiloxypropyl)-1,2,4,5,10,11-benzoperylenehexacarboxy Synthesis of acid imides
[0036]
[0037] After compound 3 was prepared according to the method described in Example 1, 0.4 g (0.5 mmol) of compound 3 was placed in a single-necked flask filled with 1 mL (3-aminopropyl)-trimethoxysilane, and stirred under argon protection After 5 minutes, the temperature was raised to 130° C. to continue the reaction for 30 hours under the protection of argon. After cooling to room temperature, the reaction mixture was dispersed in 20 mL of petroleum ether and filtered, the solid was redispersed in petroleum ether and filtered again, and the resulting compound 4 was immediately dissolved in chloroform to avoid the formation of polymers.
Embodiment 3
[0038] Example 3 N,N-bis(2,6 diisopropylphenyl)-N-(3-isopropoxypropyl)-1,2,4,5,10,11-benzoperylene hexacarboxylic acid imide Amine Synthesis
[0039]
[0040] After compound 3 was prepared according to the method described in Example 1, 0.4 g (0.5 mmol) of compound 3 was placed in a single-necked flask containing 20 mL of propionic acid, 0.6 mL (4.3 mmol) of 3-isopropoxypropylamine was added, and It was stirred for 5 min under the protection of argon, and then the temperature was raised to 130° C. to continue the reaction under the protection of argon for 30 h. After cooling to room temperature, the reaction mixture was poured into a large amount of water, filtered with suction, and the obtained solid was washed twice with water, and then washed with saturated NaHCO 3 The solution was washed twice with water until neutral, dried in a vacuum oven at 85°C and purified by silica gel column chromatography to obtain 0.41 g of a yellow solid (compound 5), with a yield of 95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum absorption wavelength | aaaaa | aaaaa |
| Molar absorptivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap