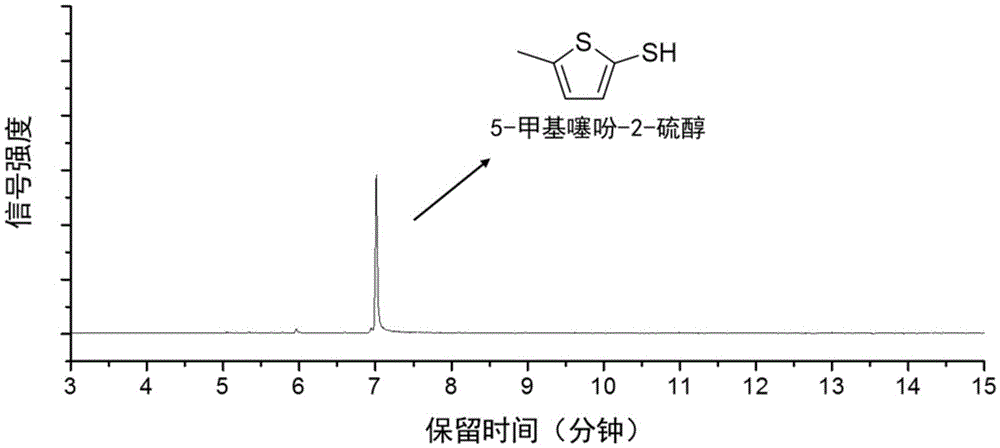
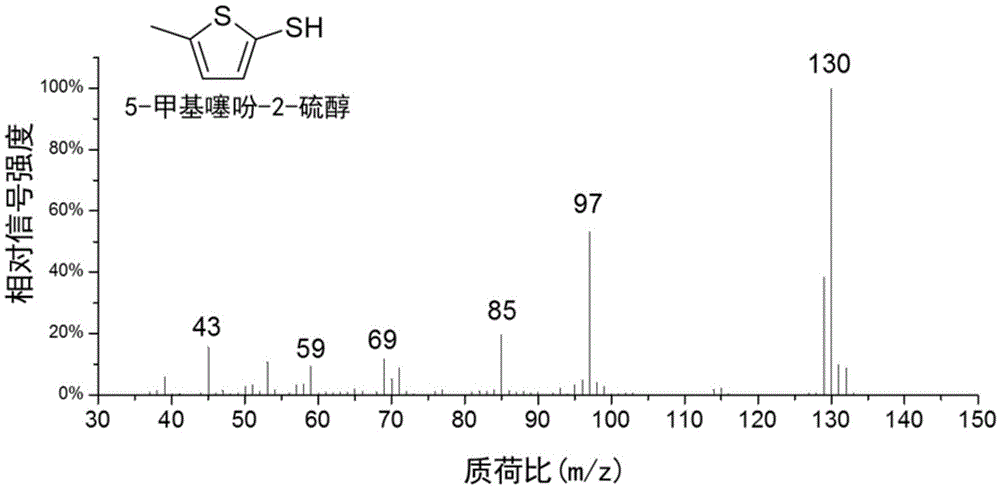
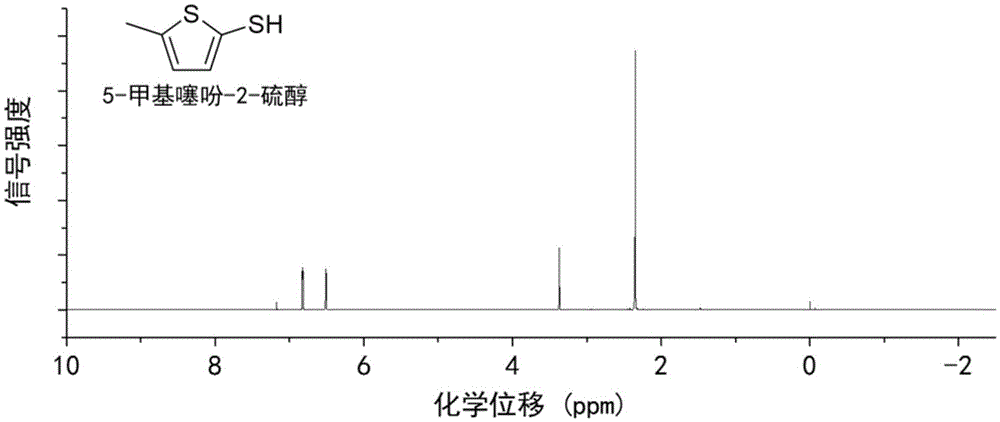
Method for preparing 5-methylthienyl-2-thiol from levulinic acid
A technology of levulinic acid and methylthiophene, applied in the direction of organic chemistry, etc., to achieve the effects of mild reaction conditions, simple steps and high product yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 0.23g of levulinic acid to 15mL of toluene, and add Lawson's reagent twice the amount of the raw material, and react at 100°C for 5h under 1bar nitrogen atmosphere to obtain a light yellow transparent liquid. The solution was washed with 2 times the volume of sodium carbonate solution with a mass concentration of 10%, and then separated by vacuum distillation to obtain 5-methylthiophene-2-thiol with a yield of 62%.
Embodiment 2
[0033] Add 0.06 g of levulinic acid to 10 mL of xylene, and add Lawson's reagent in an amount 1 times that of the raw material, and react for 1 h at 110° C. under a nitrogen atmosphere of 1 bar to obtain a light yellow transparent liquid. The solution was washed with 1 volume of sodium hydrogen carbonate solution with a mass concentration of 5%, and then separated by vacuum distillation to obtain 5-methylthiophene-2-thiol with a yield of 46%.
Embodiment 3
[0035] Add 0.6 g of levulinic acid to 20 mL of toluene, and add 1.5 times the amount of Lawson's reagent as the raw material, and react at 110 ° C for 4 h in an air atmosphere of 1 bar to obtain a light yellow transparent liquid. The liquid was washed with 3 times volume of sodium carbonate solution with a mass concentration of 7.5%, and then separated by vacuum distillation to obtain 5-methylthiophene-2-thiol with a yield of 64%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap