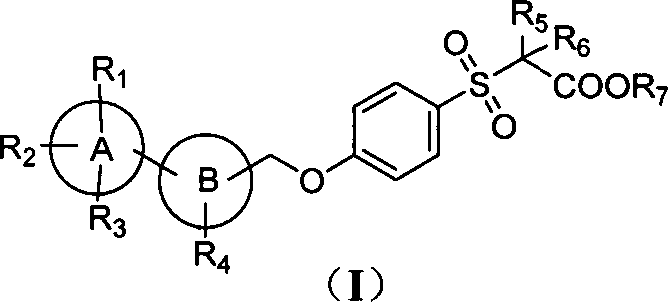
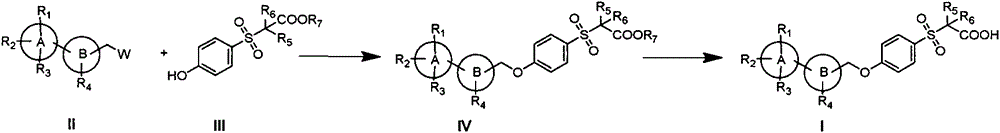
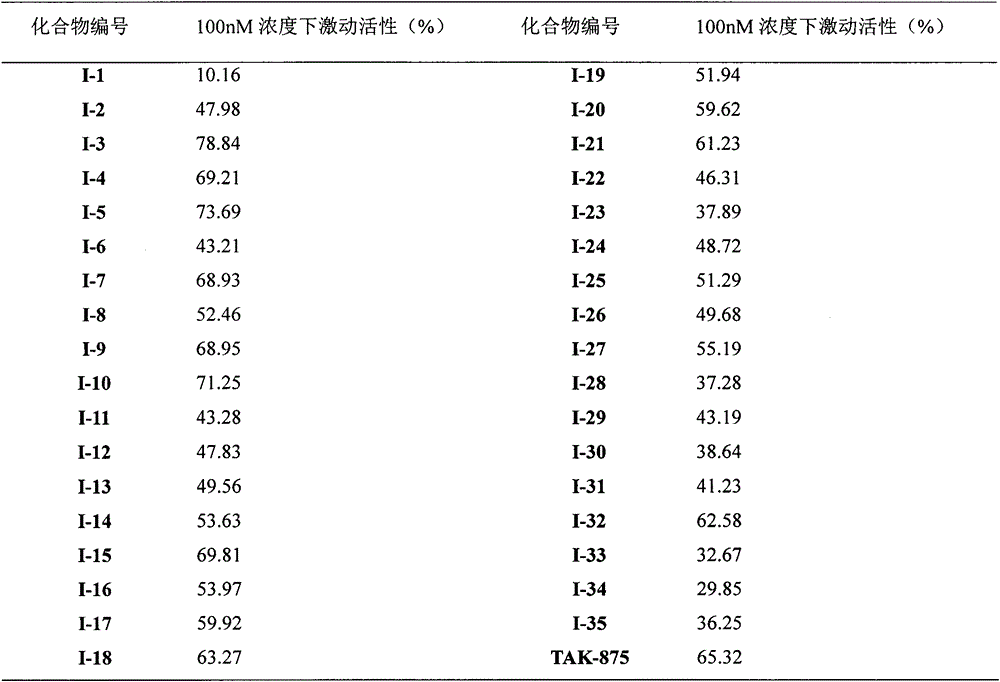
Novel sulphone acid derivative, preparation method thereof and application of novel sulphone acid derivative as medicine
A pharmacy, acetic acid technology, applied in the field of diabetes-related pharmacy, can solve the problems of hypoglycemia and weight gain, edema side effects, side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Methyl 2((4-hydroxyphenyl)sulfonyl)acetic acid
[0128]
[0129] The first step: 4-methoxythiophenol (0.88ml, 7.13mmol) was dissolved in 15ml of acetonitrile, potassium carbonate (2.96g, 21.4mmol) was added, the resulting mixture was cooled in an ice bath, and bromine was added dropwise Ethyl acetate (1.03ml, 9.27mmol) in acetonitrile solution (5ml), during the dropwise addition, the internal temperature was controlled to be lower than 0°C. After the dropwise addition, the resulting brown reaction solution was stirred at room temperature for 3 hours. After the reaction was complete as detected by TLC, add 30ml of water to dilute , extracted with ethyl acetate (30ml×4), the combined organic phases were washed successively with 1N NaOH (20ml×1), 1N HCl (20ml×1), saturated NaCl solution (20ml×2), and the resulting organic phase was washed with anhydrous sodium sulfate After drying and suction filtration, the filtrate was evaporated to remove the solvent under reduced press
Embodiment 2
[0134] Methyl 1-((4-((4'-methoxy-2',6'-dimethyl-[1,1'-biphenyl]-3-yl)methoxy)phenyl)sulfonyl ) Methyl cyclopropyl-1-acetate
[0135]
[0136] The raw material (0.2g, 0.44mmol) was dissolved in 10ml of DMF, potassium carbonate (0.15g, 1.1mmol) and a catalytic amount of TEBA were added, and after stirring at room temperature for 15min, 1,2-dibromoethane (0.10g, 0.55 mmol), the resulting reaction solution was heated at 60°C for 3 h. After the reaction was complete as detected by TLC, the reaction solution was cooled to room temperature, diluted with 30 ml of water, extracted with ethyl acetate (30 ml × 4), and the combined organic phase was washed with saturated NaCl solution (20 ml × 4). 2) Washing, the resulting organic phase was dried with anhydrous sodium sulfate, suction filtered, the filtrate was evaporated to remove the solvent under reduced pressure, and the residue was purified by column chromatography (petroleum ether / ethyl acetate, 85:25, v / v) to obtain a white solid
Embodiment 3
[0138] 2′-Chloro-[1,1′-biphenyl]-4-carbaldehyde
[0139]
[0140] Material III-1 (0.5g, 2.6mmol) was dissolved in 42ml mixed solvent (toluene / ethanol / water, 3:1:3, v / v / v), and material III-2 (0.3g, 2.6mmol) was added, Triphenylphosphopalladium (0.15g, 0.13mmol) and anhydrous sodium carbonate (0.69g, 6.5mmol) were heated at 60°C for 24h under nitrogen protection. After the reaction, cool to room temperature, dilute with 20ml of water, use diatomaceous earth as a pad for suction filtration, wash the filter cake with ethyl acetate (15ml×3), extract the filtrate with ethyl acetate (30ml×4), combine the organic phases with saturated NaCl solution (20ml×2), the obtained organic phase was dried over anhydrous sodium sulfate, suction filtered, the filtrate was evaporated to remove the solvent under reduced pressure, and the residue was subjected to column chromatography (petroleum ether / ethyl acetate, 70:30, v / v) Purification afforded 0.47 g of off-white solid with a yiel
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap