Method for preparing N-1, 5-diaryl-4-pentene-1-acetamide compound
A compound and diaryl technology, applied in pharmaceutical chemical intermediates and related chemical fields, can solve problems such as limited application, and achieve the effects of simple operation and cheap and easy-to-obtain starting materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Synthesis of trans-N-1,5-diaryl-4-pentene-1-acetamide
[0048] In a 25mL reactor, add cuprous chloride (1.49mg, 0.015mmol), silver hexafluoroantimonate (20.6mg, 0.06mmol), 1,4-bis(diphenylphosphine)butane (7.68mg, 0.018mmol ), cinnamyl alcohol (0.040g, 0.3mmol), 4-nitrobenzoic acid (10.02mg, 0.06mmol), after nitrogen replacement 3 times, add 0.3mL acetonitrile, after stirring at room temperature for 10 minutes, add styrene (0.094 g, 0.9 mmol), stirred at 105°C for 16 hours. Column chromatography separation (silica gel, 200-300 mesh; developer, petroleum ether / ethyl acetate = 3 / 2) to obtain trans-N-1,5-diaryl-4-pentene-1-acetamide 0.069 g, yield 82%.
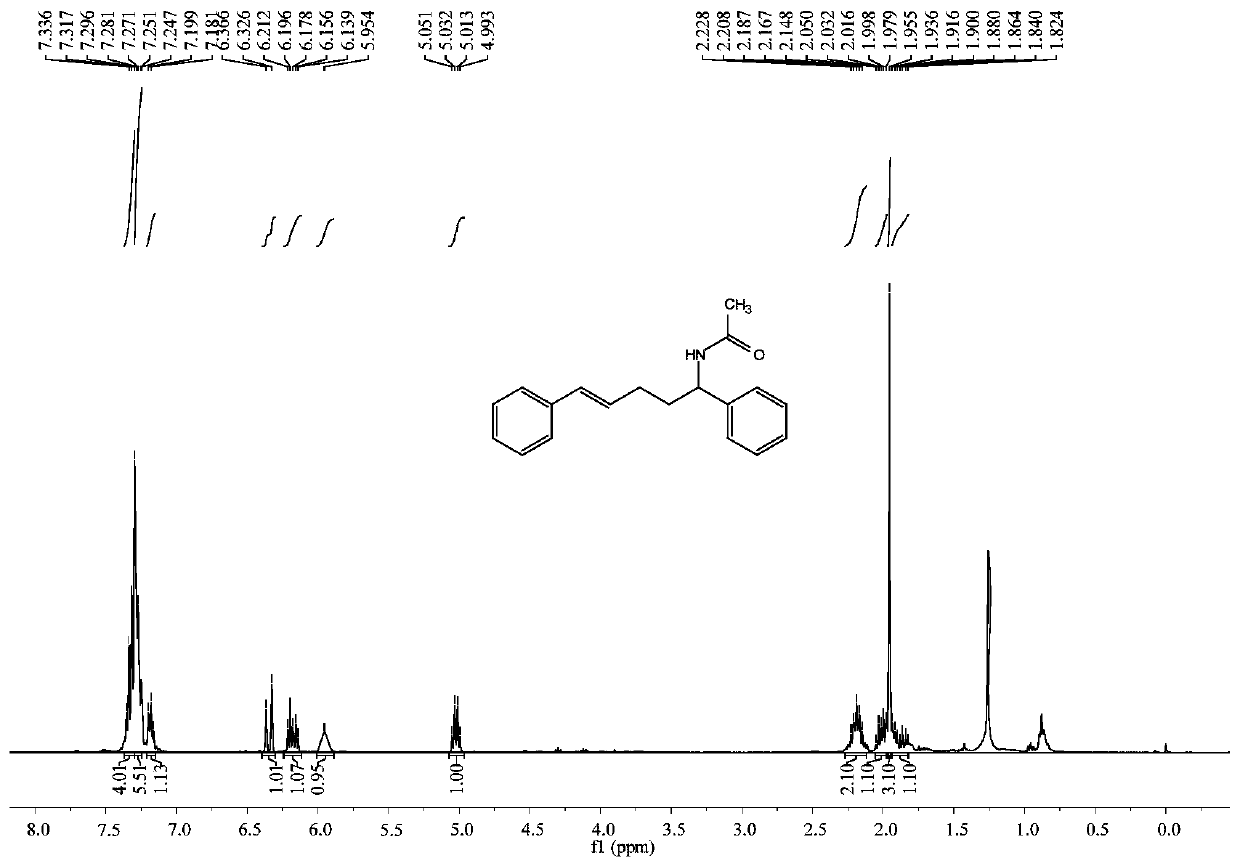
[0049] trans-N-1,5-diaryl-4-pentene-1-acetamide white solid; 1 H NMR (400MHz, CDCl 3 ):δ7.35–7.32(m,4H),7.30–7.25(m,5H),7.18(t,J=6.9Hz,1H),6.35(d,J=15.9Hz,1H),6.18(dt, J=15.9,6.8Hz,1H),5.95(bs,1H),5.02(q,J=7.6Hz,1H),2.23–2.15(m,2H),2.05–1.98(m,1H),1.96(s ,3H),1.94–1.82(m,1H). 13 C NMR (100MHz, CDCl 3
Embodiment 2
[0050] Example 2: Synthesis of trans-N-5-(4-bromophenyl)-1-phenyl-4-pentene-1-acetamide
[0051] Operation is the same as in Example 1, by reacting trans-3-(4-bromophenyl)-2-propen-1-alcohol and styrene in acetonitrile to obtain trans-N-5-(4-bromophenyl)- 0.086 g of 1-phenyl-4-pentene-1-acetamide, yield 80%.
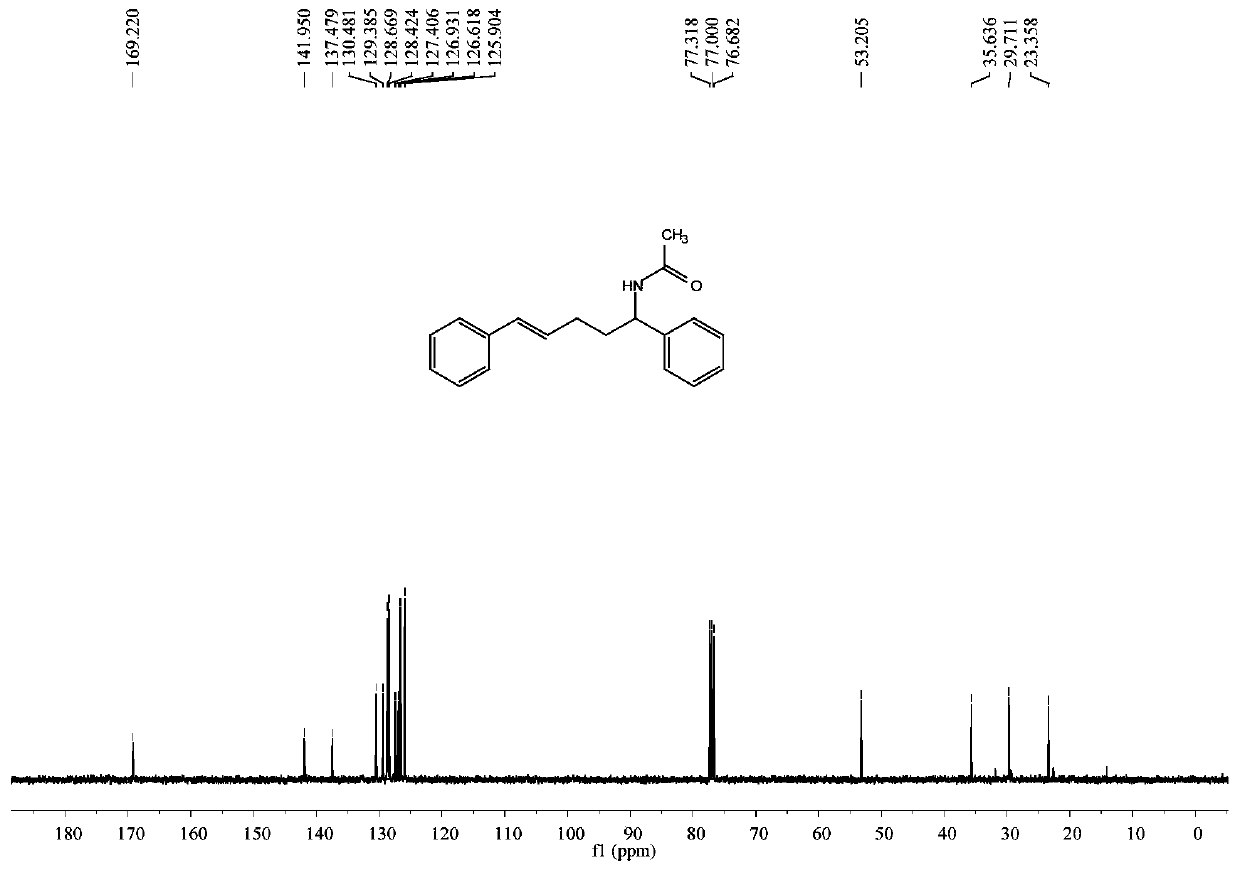
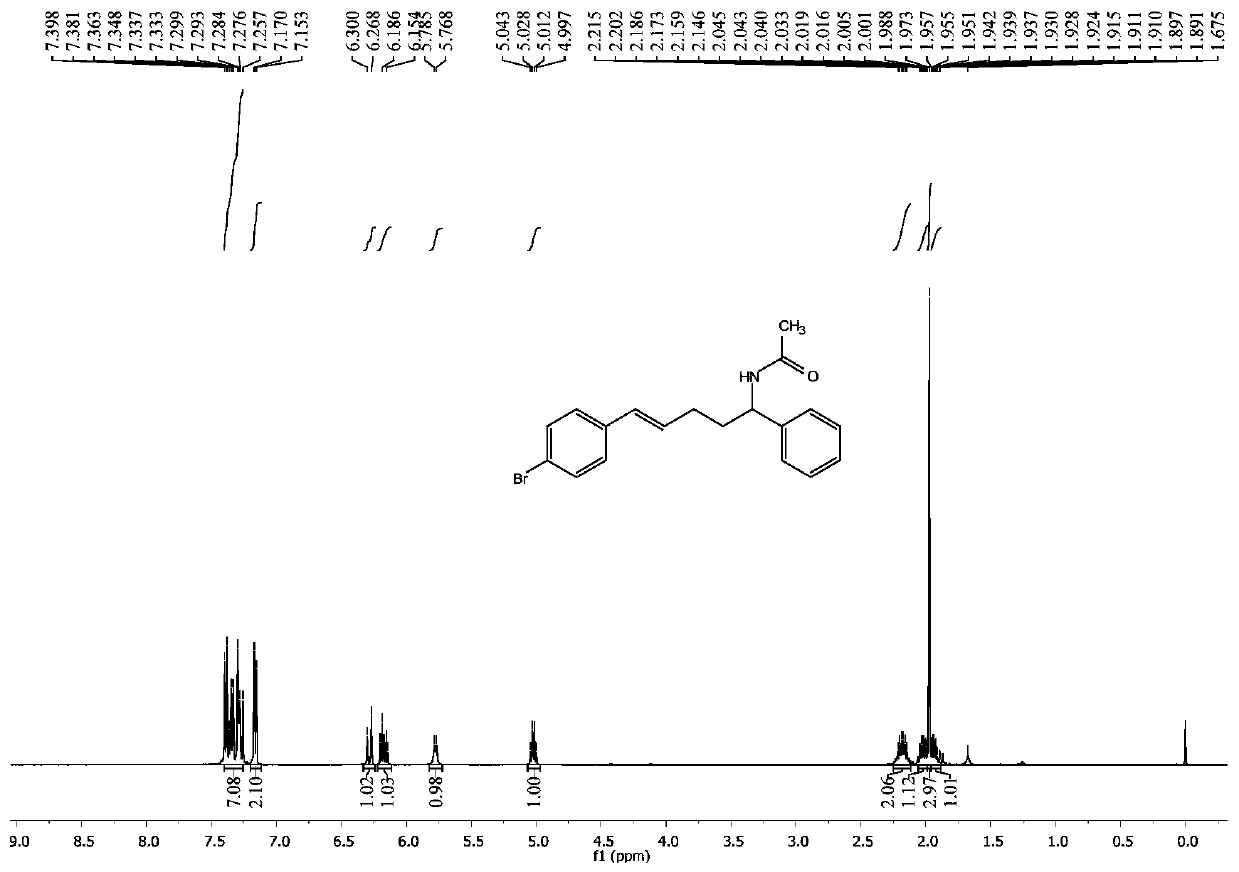
[0052] trans-N-5-(4-bromophenyl)-1-phenyl-4-pentene-1-acetamide yellow solid; 1 H NMR (500MHz, CDCl 3 ):δ7.40–7.26(m,7H),7.16(d,J=8.4Hz,2H),6.28(d,J=15.9Hz,1H),6.17(dt,J=15.9,6.7Hz,1H) ,5.78(d,J=7.7Hz,1H),5.02(q,J=7.6Hz,1H),2.22–2.15(m,2H),2.05–1.99(m,1H),1.97(s,3H), 1.96–1.89(m,1H). 13 C NMR (125MHz, CDCl 3 ):δ169.2,141.8,136.5,131.5,130.3,129.4,128.8,127.6,127.5,126.7,120.6,53.2,35.5,29.7,23.5.IR(KBr):3256,3069,2924,2852,1950,14945, ,1486,1278,1178,1058,1030,966,700,640cm -1 .HRMS(EI):calcd for C 19 h 20 BrNO[M] + :357.0728, and 359.0708 Found 357.0723 and 359.0701.
Embodiment 3
[0053] Example 3: Synthesis of trans-N-1-phenyl-5-(o-tolyl)-4-pentene-1-acetamide
[0054] Operation is the same as in Example 1, by reacting trans-3-(o-tolyl)-2-propen-1-alcohol and styrene in acetonitrile to obtain trans-N-1-phenyl-5-(o-tolyl) -4-pentene-1-acetamide 0.066g, yield 75%.
[0055] trans-N-1-phenyl-5-(o-tolyl)-4-pentene-1-acetamide brown solid; 1 H NMR (400MHz, CDCl 3 ):δ7.36–7.25(m,6H),7.15–7.10(m,3H),6.54(d,J=15.8Hz,1H),6.05(dt,J=15.8,6.8Hz,1H),5.90( bs,1H),5.04(q,J=7.6Hz,1H),2.32(s,3H),2.31–2.17(m,2H),2.05–2.00(m,1H),1.97(s,3H),1.95 –1.89(m,1H). 13 C NMR (125MHz, CDCl 3 ): δ169.0, 138.9, 137.6, 137.2, 130.5, 129.5, 129.4, 128.5, 126.9, 126.6, 126.0, 53.0, 35.6, 29.8, 23.5, 21.0.IR(KBr): 3260, 3047, 3033, 2915, 2951, ,1644,1540,1409,1250,1178,1031,967,742,701cm -1 .HRMS(EI):C 20 h 23 NO[M] + :293.1780, Found293.1789.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap