Method for preparing heteroaryl cyanide through cyanation reaction on palbociclib intermediate
A cyanation reaction, the technology of Palbocoxib, which is applied in the field of preparing heteroaryl cyanide by cyanation reaction of Palbocoxib intermediates, can solve the problems of high reaction cost, low yield, harmfulness to human body and environment, etc. , to achieve the effect of low raw material cost, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
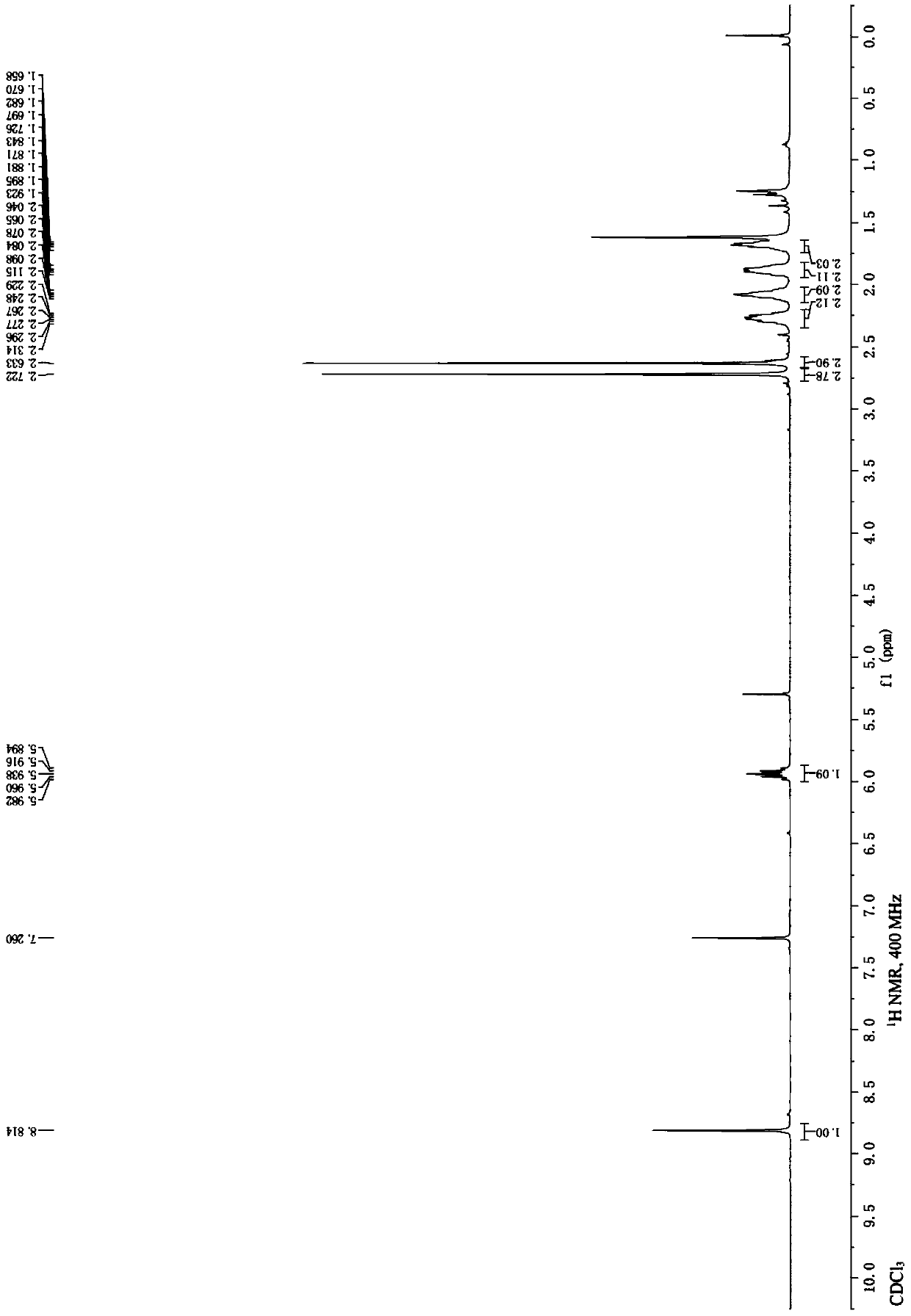
Embodiment 1
[0028] According to the following chemical reaction equation, the cyanation of 6-bromo-8-cyclopentyl-5-methyl-2-methylthiopyrido[2,3-d]pyrimidin-7-one prepared 6-cyano- 8-Cyclopentyl-5-methyl-2-methylthiopyrido[2,3-d]pyrimidin-7-one:
[0029]
[0030] Under the protection of inert gas, 6-bromo-8-cyclopentyl-5-methyl-2-methylsulfanylpyrido[2,3-d]pyrimidin-7-one (4,500mg, 1.41 mmol), potassium hexacyanoferrite trihydrate (298 mg, 0.71 mmol), bis[2-(diphenylphosphino)phenyl]ether palladium chloride (303 mg, 0.42 mmol), potassium acetate (69.3 mg, 0.71 mmol), N,N-dimethylformamide (5 mL) and water (0.5 mL), the reaction flask was sealed with a rubber stopper and heated and stirred at 100° C. for 7 hours. After the reaction solution was cooled, ethyl acetate was added, and then washed with saturated brine. The obtained organic phase was dried over anhydrous sodium sulfate, filtered and concentrated. The light yellow solid obtained after the crude product was subjected to column ch
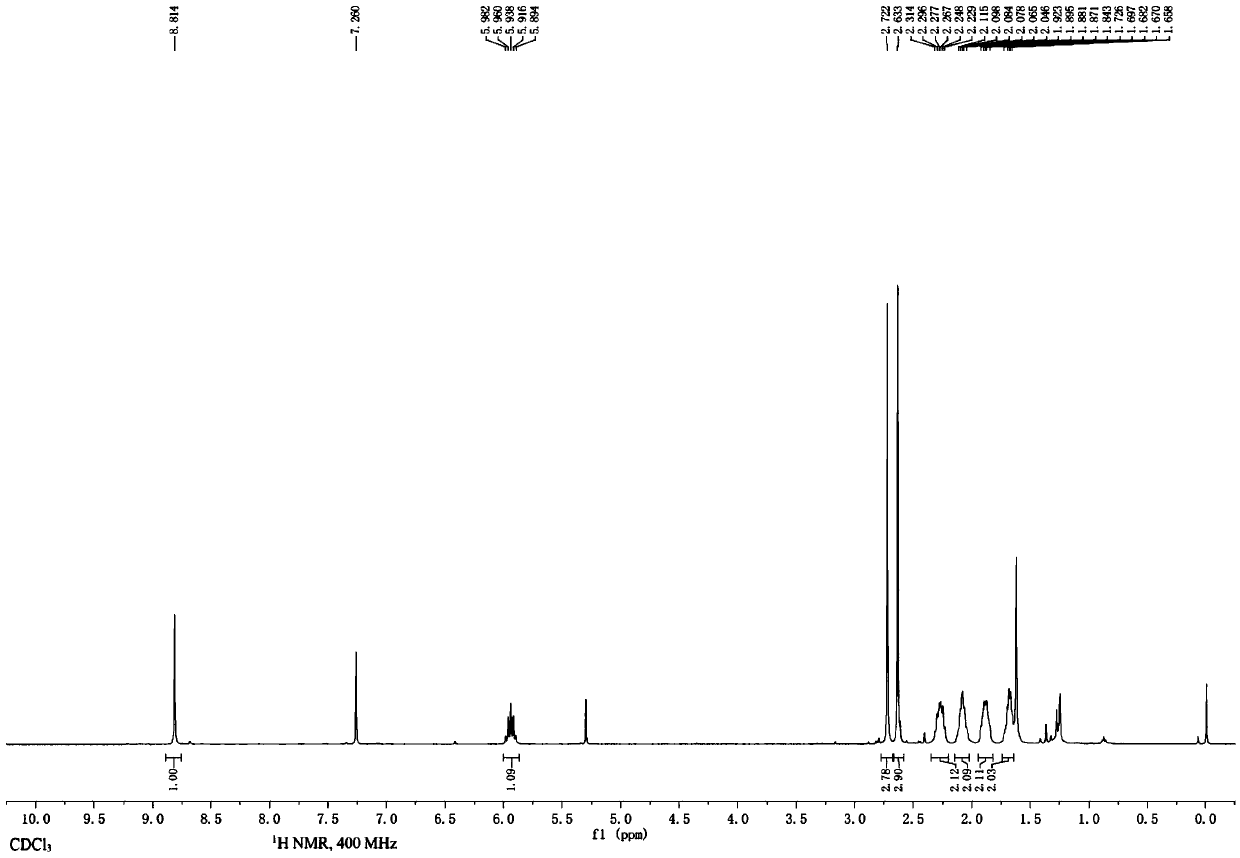
Embodiment 2
[0033] According to the following chemical reaction equation, 6-cyano-8-cyclopentyl was prepared by cyanation of 6-bromo-8-cyclopentyl-2-methylthiopyrido[2,3-d]pyrimidin-7-one -2-Methylthiopyrido[2,3-d]pyrimidin-7-one:
[0034]
[0035] Under the protection of inert gas, 6-bromo-8-cyclopentyl-2-methylthiopyrido[2,3-d]pyrimidin-7-one (4a, 680.5 mg, 2 mmol) was added to a 25 mL reaction flask, Potassium hexacyanoferrite trihydrate (422.4 mg, 1 mmol), bis[2-(diphenylphosphino)phenyl]ether palladium chloride (429.5 mg, 0.6 mmol), potassium acetate (98.1 mg, 1 mmol) ), N,N-dimethylformamide (7 mL) and water (0.7 mL), the reaction flask was sealed with a rubber stopper and heated and stirred at 100° C. for 7 hours. After the reaction solution was cooled, ethyl acetate was added, and then washed with saturated brine. The obtained organic phase was dried over anhydrous sodium sulfate, filtered and concentrated. The light yellow solid obtained after the crude product was subjected to co
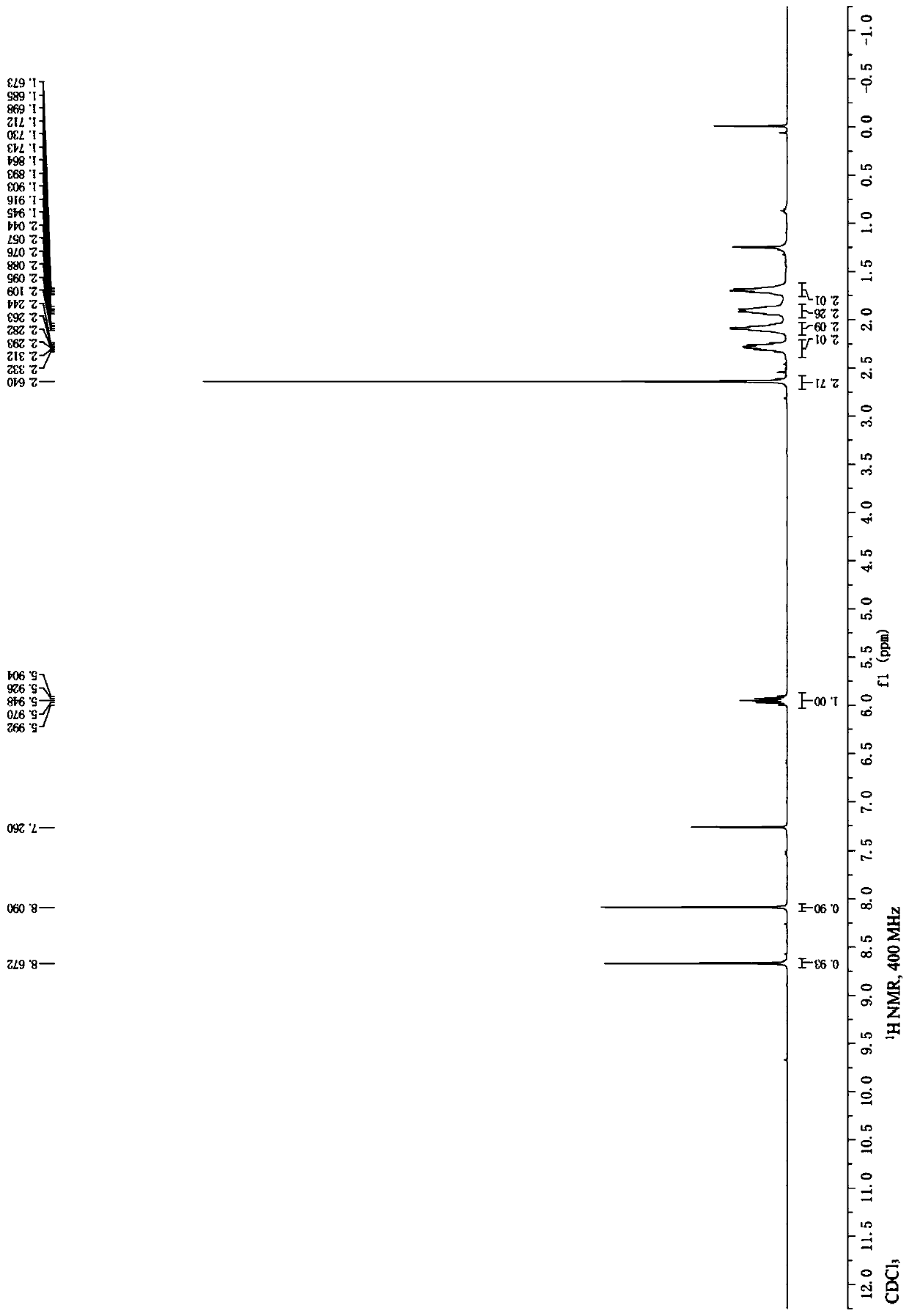
Embodiment 3
[0038] According to the following chemical reaction equation, 4-(6-(6-Bromo-8-cyclopentyl-5-methyl-7-oxo-7,8-dihydropyrido[2,3-d]pyrimidine-2 -ylamino)pyridin-3-yl)piperazine-1-carboxylic acid tert-butyl ester cyanation to prepare 4-(6-(6-cyano-8-cyclopentyl-5-methyl-7-oxo) -7,8-Dihydropyrido[2,3-d]pyrimidin-2-ylamino)pyridin-3-yl)piperazine-1-carboxylic acid tert-butyl ester:
[0039]
[0040] Under the protection of inert gas, 4-(6-(6-bromo-8-cyclopentyl-5-methyl-7-oxo-7,8-dihydropyrido[2,3] was added to a 25 mL reaction flask. -d] pyrimidin-2-ylamino)pyridin-3-yl)piperazine-1-carboxylate tert-butyl ester (4b, 584.5 mg, 1 mmol), potassium hexacyanoferrite trihydrate (211.2 mg, 0.5 mmol) ), bis[2-(diphenylphosphino)phenyl]ether palladium chloride (214.8 mg, 0.3 mmol), potassium acetate (49.1 mg, 0.5 mmol), N,N-dimethylformamide (4 mL) and water (0.4 mL), the reaction vial was sealed with a rubber stopper and heated and stirred at 100°C for 7 hours. After the reaction solut
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap