Synthesis method of 7-mercaptoisobenzofuran-1(3H)-one compound
A ketone compound and synthesis method technology, applied in the field of pesticide synthesis, can solve the problems of high cost, potential safety hazards, low purity of the target product, etc., reduce production operations and energy consumption, reduce the generation of three wastes, and be suitable for industrial production. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
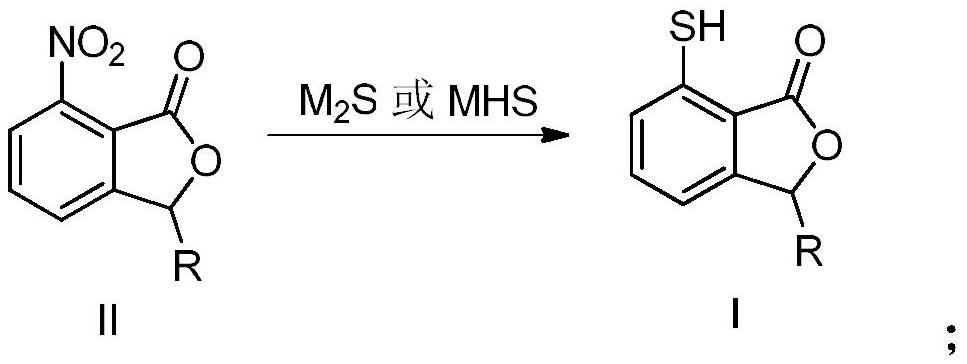
[0031] The synthetic method of 7-mercapto-3-methylisobenzofuran-1 (3H)-ketone, the steps are as follows:
[0032] (1) Add compound II and sodium sulfide to DMF, react at 15°C for 5 hours, filter, and evaporate the filtrate to remove the solvent under reduced pressure to obtain a brown solid-liquid mixture;
[0033] Among them, compound II is
[0034] The molar ratio of the compound II and sodium sulfide is 1:1.1;
[0035] (2) Add 30mL water, 30mL methylene chloride and 80wt% acetic acid to the brown solid-liquid mixture gained in step (1), adjust the pH to 5-6, filter, separate liquids, and evaporate the organic solvent under reduced pressure in the organic phase to obtain light Yellow oil, namely 7-mercapto-3-methylisobenzofuran-1(3H)-one; the HPLC area-normalized purity of the product in the reaction solution was 80.71%, and the yield was 82%.
[0036] Analyze the resulting product, 1 H NMR (400MHz, CDCl 3 )δ7.46(t, J=7.7Hz, 1H), 7.28(d, J=7.8Hz, 1H), 7.10(d, J=7.5Hz, 1H)
Embodiment 2
[0038] The synthetic method of 7-mercapto-3-methylisobenzofuran-1 (3H)-ketone, the steps are as follows:
[0039] (1) Add compound II, sodium sulfide and elemental sulfur to DMF, react at 25°C for 2 hours, filter, and distill the filtrate to remove the solvent under reduced pressure to obtain a brown solid-liquid mixture;
[0040] Among them, compound II is
[0041] The molar ratio of compound II, sodium sulfide and elemental sulfur is 1:1.5:1.65;
[0042] (2) Add 30mL water, 30mL methylene chloride and 80wt% acetic acid to the brown solid-liquid mixture gained in step (1), adjust the pH to be 5-6, filter, separate liquids, and evaporate the organic solvent under reduced pressure in the organic phase to obtain light Yellow oil, namely 7-mercapto-3-methylisobenzofuran-1(3H)-one; the HPLC area-normalized purity of the product in the reaction solution was 88.29%, and the yield was 90%.
Embodiment 3
[0044] The synthetic method of 7-mercapto-3-methylisobenzofuran-1 (3H)-ketone, the steps are as follows:
[0045] (1) Add compound II, sodium sulfide and elemental sulfur to DMF, react at 0°C for 5 hours, filter, and evaporate the filtrate to remove the solvent under reduced pressure to obtain a brown solid-liquid mixture;
[0046] Among them, compound II is
[0047] The molar ratio of compound II, sodium sulfide and elemental sulfur is 1:1.5:1.65;
[0048] (2) Add 30mL water, 30mL methylene chloride and 80wt% acetic acid to the brown solid-liquid mixture gained in step (1), adjust the pH to be 5-6, filter, separate liquids, and evaporate the organic solvent under reduced pressure in the organic phase to obtain light Yellow oil, namely 7-mercapto-3-methylisobenzofuran-1(3H)-one; the HPLC area-normalized purity of the product in the reaction solution was 80.99%, and the yield was 85%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap