Method for synthesizing C-5-bit trifluoromethylated 8-aminoquinoline by utilizing micro-channel reaction device under visible light
A technology of microchannel reaction and trifluoromethylation, which is applied in organic chemistry and other fields, can solve problems such as low yield and atom utilization, high requirements for reactor design, poor selectivity, etc., and achieve shortened reaction time and improved Yield, the effect of reducing side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
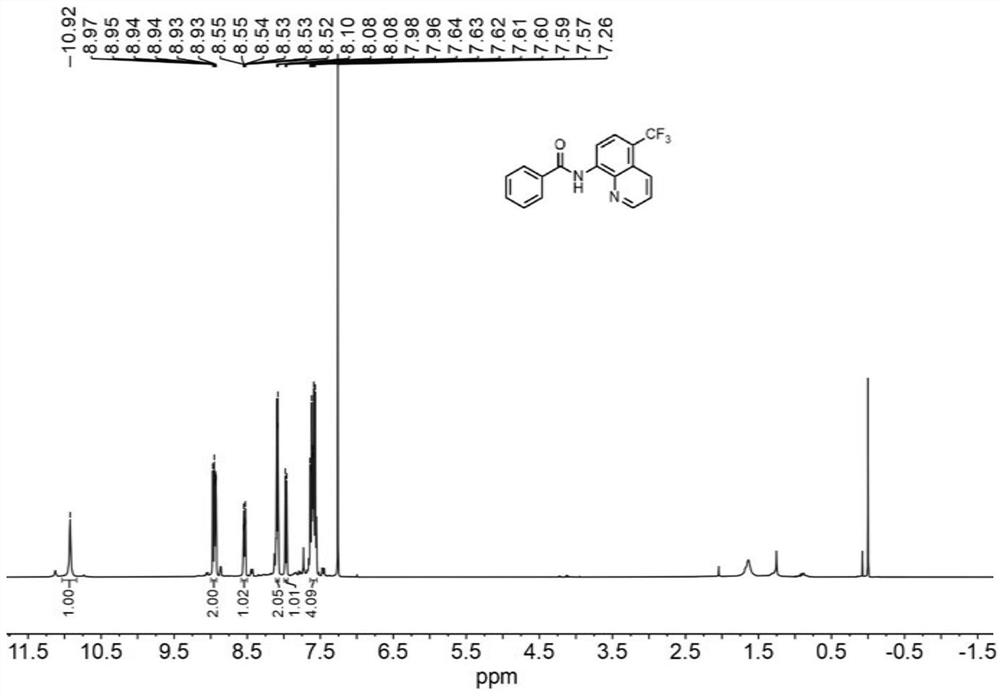
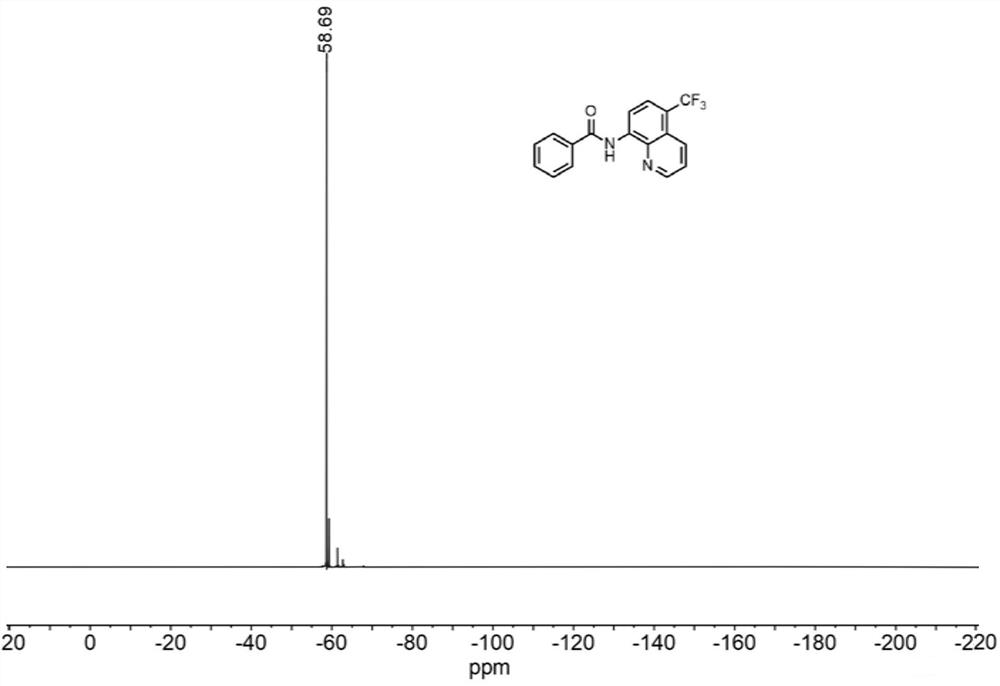
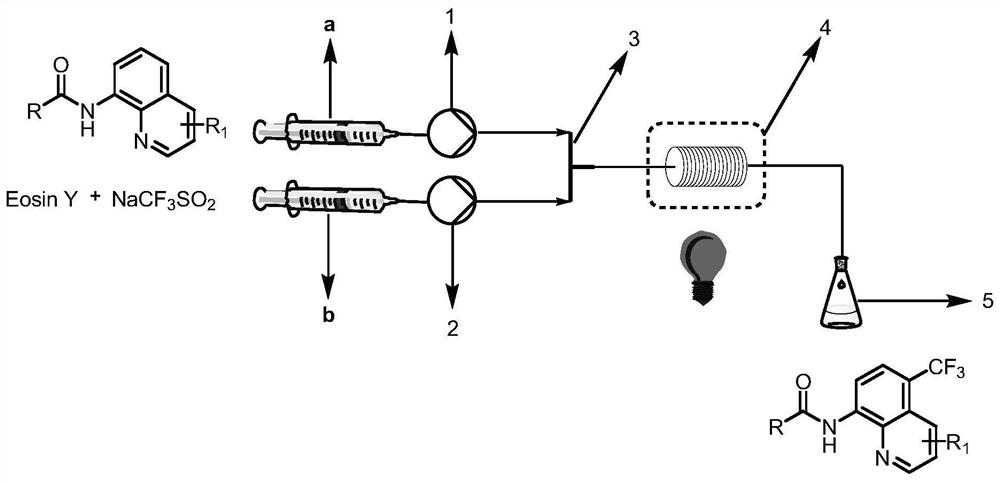
[0043] N-(quinolin-8-yl)benzamide (5 mmol, 1.0 equiv) was weighed and dissolved in dimethylformamide (10 mL) and loaded into syringe a. NaCF 3 SO 2(10 mmol, 2.0 equiv) and Eosin Y (0.25 mmol, 5% equiv) were dissolved in water (10 mL) and loaded in syringe b. The reaction solution in a and b enters the reactor with a coil inner diameter of 0.5mm under visible light through the Y-type mixer, the flow rate of the syringe a and b is 0.5mL / min, and the flow rate of the microreactor is 1.0mL / min, the reaction temperature was controlled at 30°C, the residence time was 20min, and the reaction progress was detected by TLC (petroleum ether:ethyl acetate=4:1). 100mL saturated NaHCO 3 (aq) washing, liquid separation, the aqueous phase was extracted with ethyl acetate (150mL×3), the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and the crude product was concentrated with ethyl acetate / n-hexane as solvents respectively Recrystallized
Embodiment 2
[0045] N-(quinolin-8-yl)benzamide (5 mmol, 1.0 equiv) was weighed and dissolved in dimethyl sulfoxide (10 mL) and loaded into syringe a. NaCF 3 SO 2 (10 mmol, 2.0 equiv) and Eosin Y (0.25 mmol, 5% equiv) were dissolved in water (10 mL) and loaded in syringe b. The reaction solution in a and b enters the reactor with a coil inner diameter of 0.5mm under visible light through the Y-type mixer, the flow rate of the syringe a and b is 0.5mL / min, and the flow rate of the microreactor is 1.0mL / min, the reaction temperature was controlled at 30°C, the residence time was 20min, and the reaction progress was detected by TLC (petroleum ether:ethyl acetate=4:1). 100mL saturated NaHCO 3 (aq) washing, liquid separation, the aqueous phase was extracted with ethyl acetate (150mL×3), the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and the crude product was concentrated with ethyl acetate / n-hexane as solvents respectively Recrystalliz
Embodiment 3
[0047] N-(quinolin-8-yl)benzamide (5 mmol, 1.0 equiv) was weighed and dissolved in dichloromethane (10 mL) and loaded into syringe a. NaCF 3 SO 2 (10 mmol, 2.0 equiv) and Eosin Y (0.25 mmol, 5% equiv) were dissolved in water (10 mL) and loaded in syringe b. The reaction solution in a and b enters the reactor with a coil inner diameter of 0.5mm under visible light through the Y-type mixer, the flow rate of the syringe a and b is 0.5mL / min, and the flow rate of the microreactor is 1.0mL / min, the reaction temperature was controlled at 30°C, the residence time was 20min, and the reaction progress was detected by TLC (petroleum ether:ethyl acetate=4:1). 100mL saturated NaHCO 3 (aq) washing, liquid separation, the aqueous phase was extracted with ethyl acetate (150mL×3), the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and the crude product was concentrated with ethyl acetate / n-hexane as solvents respectively Recrystallized
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap