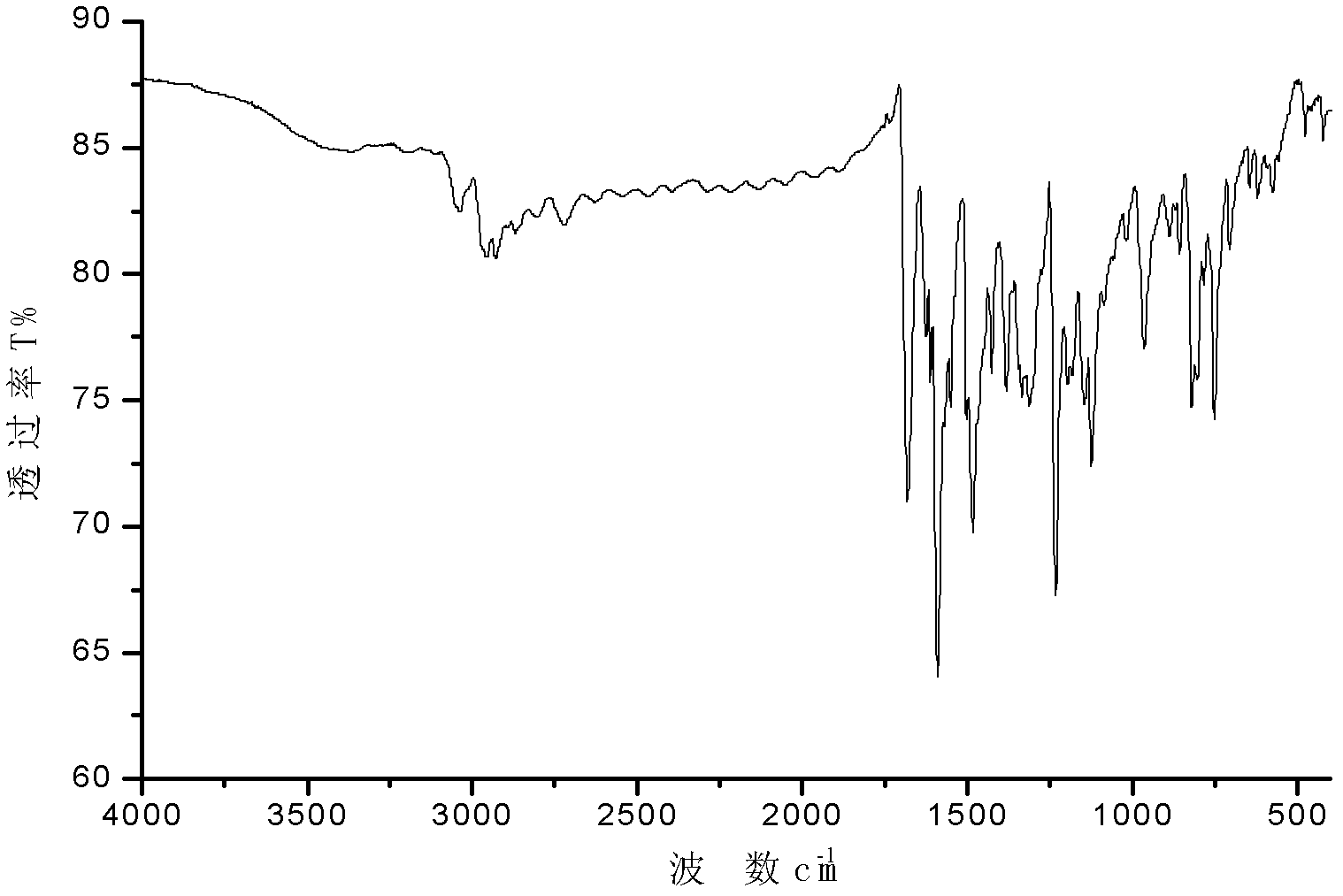
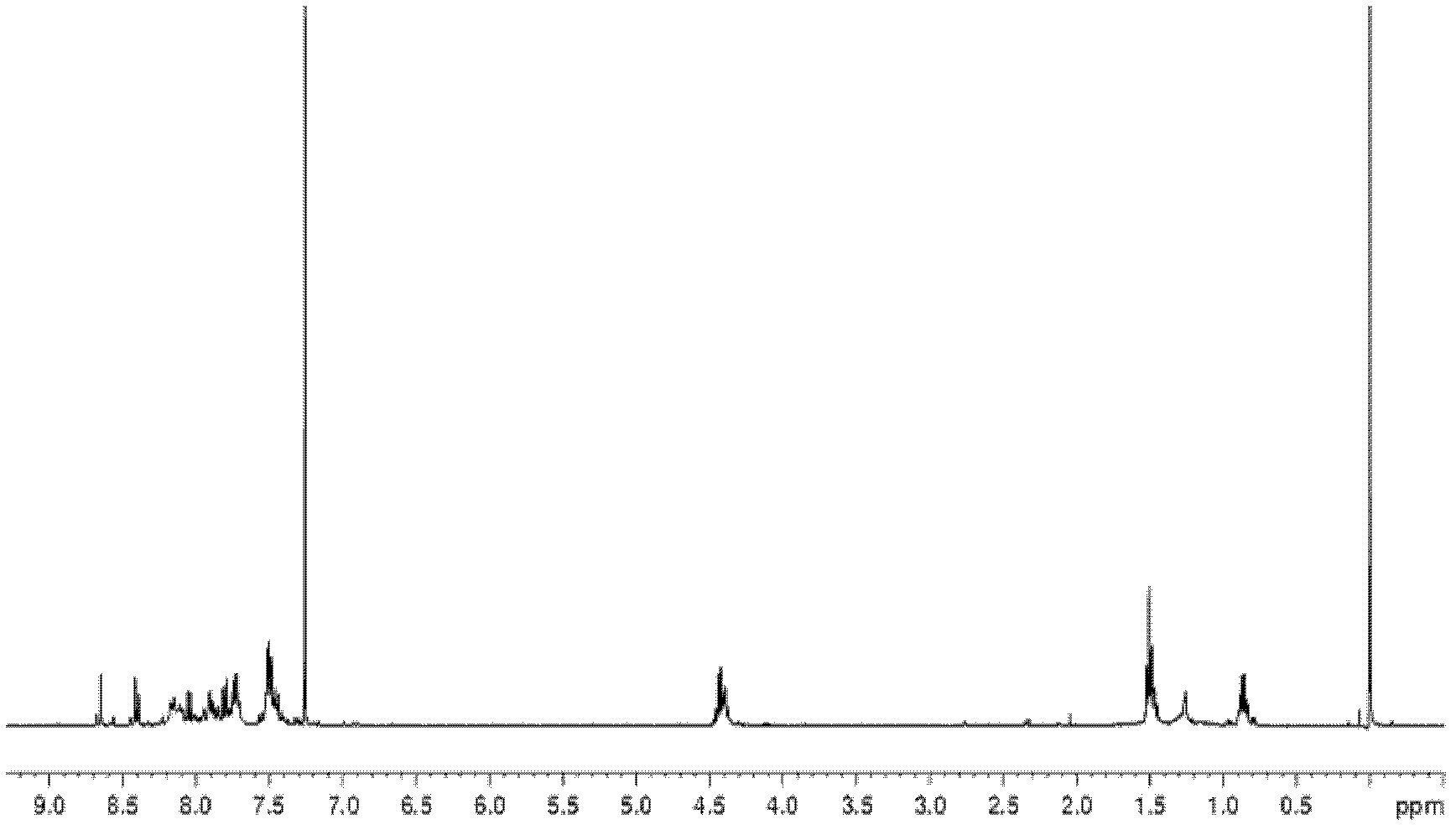
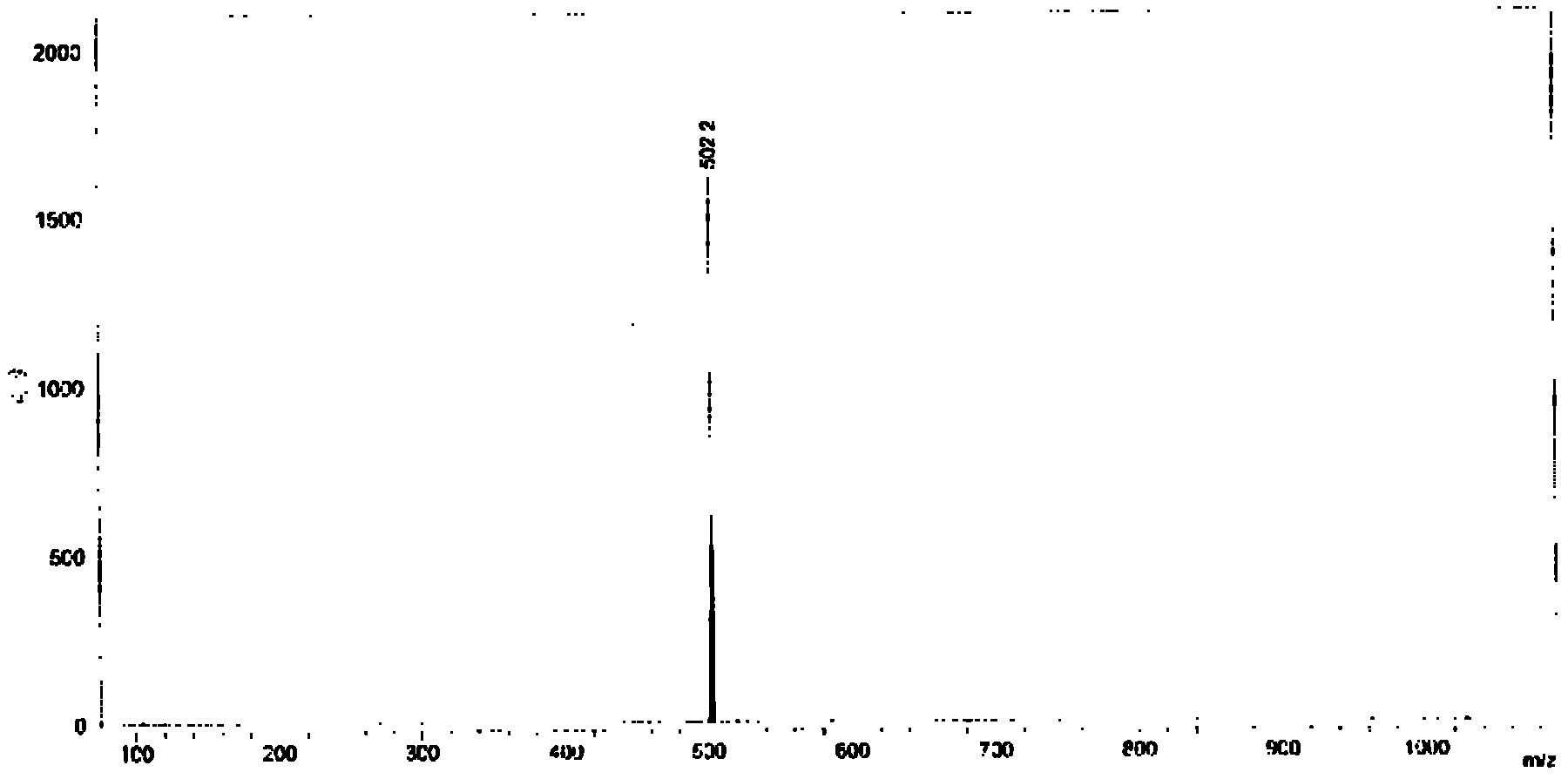
N-alkyl group-3, 6-di-(2-quinoline) vinyl) carbazole and preparation method thereof
A vinyl, methyl quinoline technology, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve the problems of poor thermal stability and photochemical stability, and achieve good photochemical stability, good thermal stability and The effect of photochemical stability and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0026] Specific embodiment one: the N-alkyl-3 of the present embodiment, the structural formula of 6-two [(2-quinoline) vinyl] carbazole is as follows:
[0027]
[0028] Among them, R is C n h 2n+1 Or benzyl, n is 1-8.
[0029] The N-alkyl-3,6-bis[(2-quinoline)vinyl]carbazole of the present embodiment is that the electron acceptor group is connected to both sides of the carbazole ring at the 3,6 position, and the 3, 6-diformyl-N-alkyl-carbazole derivatives and 2-methylquinoline are produced by Knoevenagel Reaction under the condition of acetic anhydride as condensing agent. It can produce fluorescence phenomenon under the irradiation conditions of 365nm of ordinary 8 watts and 254nm low-energy ultraviolet wavelength of 6 watts, and the N-alkyl-3,6-bis[(2-quinoline)ethylene of this embodiment Base] carbazole has good thermal stability and photochemical stability, and can undergo two-photon absorption phenomenon under 800nm femtosecond laser, and can be excited to produce f
specific Embodiment approach 2
[0030] Specific embodiment two: N-alkyl-3 of the present embodiment, the preparation method of 6-two [(2-quinoline) vinyl] carbazole is carried out according to the following steps: 1. Weigh 1% by mass percentage ~3% of 3,6-diformyl N-alkyl-carbazole, 5%~7% of 2-methylquinoline and 90%~94% of acetic anhydride; , mix 6-diformyl-N-alkyl-carbazole and 2-methylquinoline evenly, then add the acetic anhydride weighed in step 1, stir evenly, heat to 100°C~140°C, and keep for 24~ 72h, cooled to room temperature to obtain the reaction solution; 3. Mix the reaction solution obtained in step 2 with water in a volume ratio of 1:2 to obtain the initial mixed solution; then use a concentration of 10-25mol / L alkaline The solution adjusts the pH value of the initial mixed solution to 7.0 to obtain a mixed solution; 4. Extract the mixed solution obtained in step 3 with dichloromethane, collect the extract, and use a rotary evaporator to store the extract at a temperature of 50°C to 70°C Carry
specific Embodiment approach 3
[0034] Embodiment 3: This embodiment is different from Embodiment 2 in that: the water described in step 3 is pure water. Others are the same as in the second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap