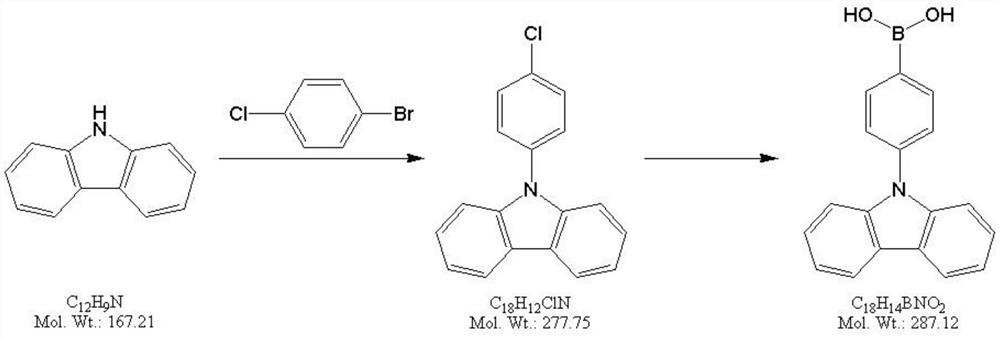
Preparation method of 4-(9H-carbazol-9-yl)phenylboronic acid
A technology of phenylboronic acid and carbazole, which is applied in the field of preparation of 4-phenylboronic acid, can solve problems such as not being suitable for industrial production, and achieve the effect of avoiding ultra-low temperature reaction and being suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Preparation of 9-(4-chlorophenyl)-9H-carbazole
[0023] Add 100g carbazole to a 1L three-necked flask, then add 500ml N,N-dimethylformamide, stir to dissolve; then add 137.4g 4-chlorobromobenzene, 206.6g potassium carbonate, 5.0g Pd(dppf) 2 Cl 2 , under the protection of nitrogen, the temperature was raised to 100°C for reaction, and after 8 hours TLC monitored the complete reaction of carbazole; after the reaction, the reaction solution was lowered to 25°C, filtered, and 500ml of water and 500ml of ethyl acetate were added to the filtrate to extract and separate the liquid; water The phase was extracted once more with 200ml ethyl acetate, and the organic phases were combined; the organic phase was washed 3 times with 500ml×3 saturated brine, dried over anhydrous sodium sulfate, and then evaporated to dryness to obtain a brown viscous substance; Add 800ml of toluene to the mixture, heat to reflux to dissolve, then evaporate 500ml of toluene under normal pressure, cool
Embodiment 2
[0030] (1) Preparation of 9-(4-chlorophenyl)-9H-carbazole
[0031] Add 2.00kg carbazole to a 20L glass kettle, then add 10L N,N-dimethylformamide, stir to dissolve; then add 3.43kg 4-chlorobromobenzene, 4.96g potassium carbonate, 100g Pd(dppf) 2 Cl 2 , under the protection of nitrogen, the temperature was raised to 100°C for reaction, and after 10 hours, TLC monitored the complete reaction of carbazole; after the reaction, the reaction solution was lowered to 25°C, filtered, and 10L of water and 10L of ethyl acetate were added to the filtrate for extraction and liquid separation; water The phase was extracted once more with 5L ethyl acetate, and the organic phases were combined; the organic phase was washed 3 times with 12L×3 saturated brine, dried over anhydrous sodium sulfate, and then evaporated to dryness to obtain a brown viscous substance; Add 11L of toluene to the mixture, heat to reflux to dissolve, then evaporate 4L of toluene under normal pressure, cool down to 0-10°C
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap