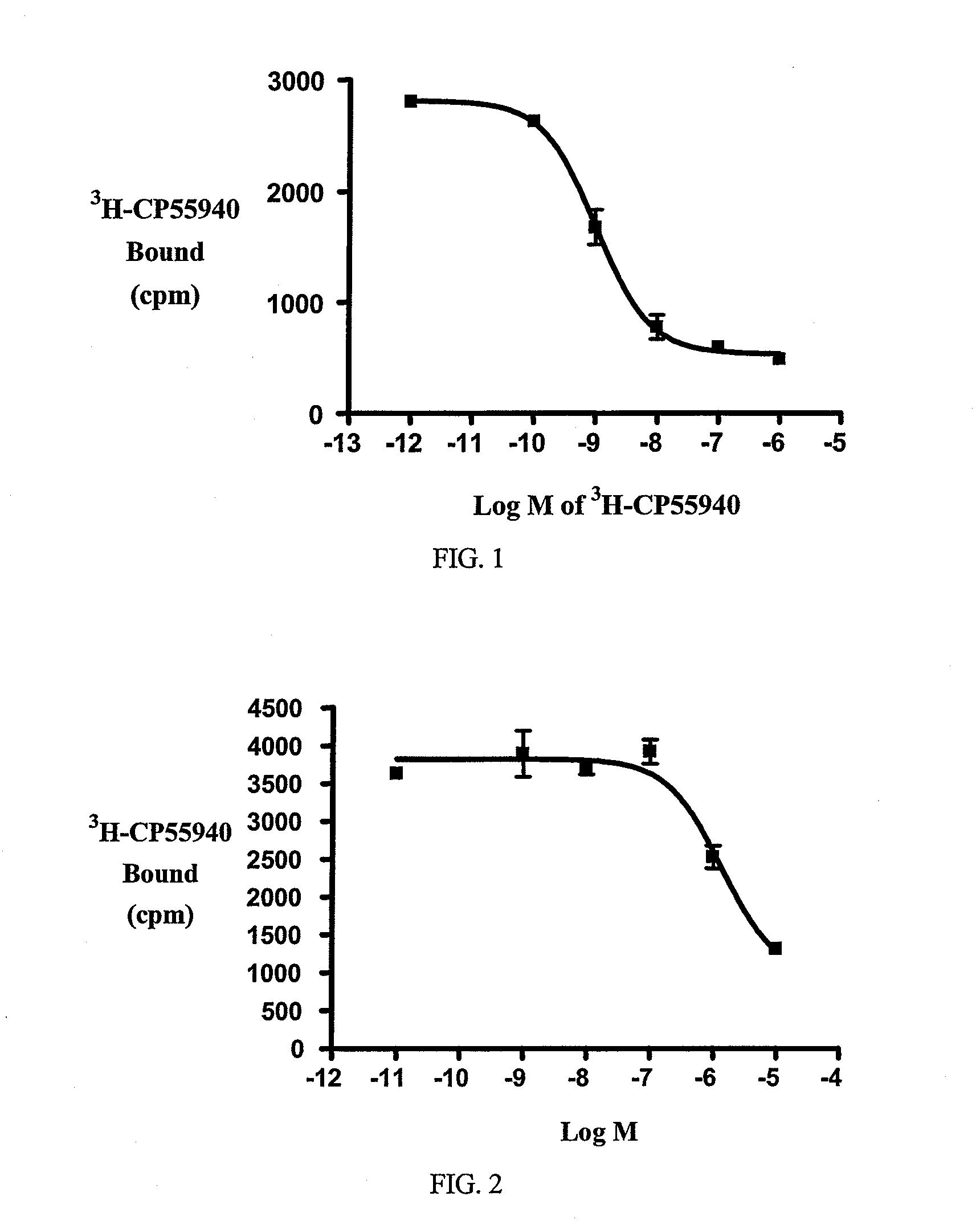
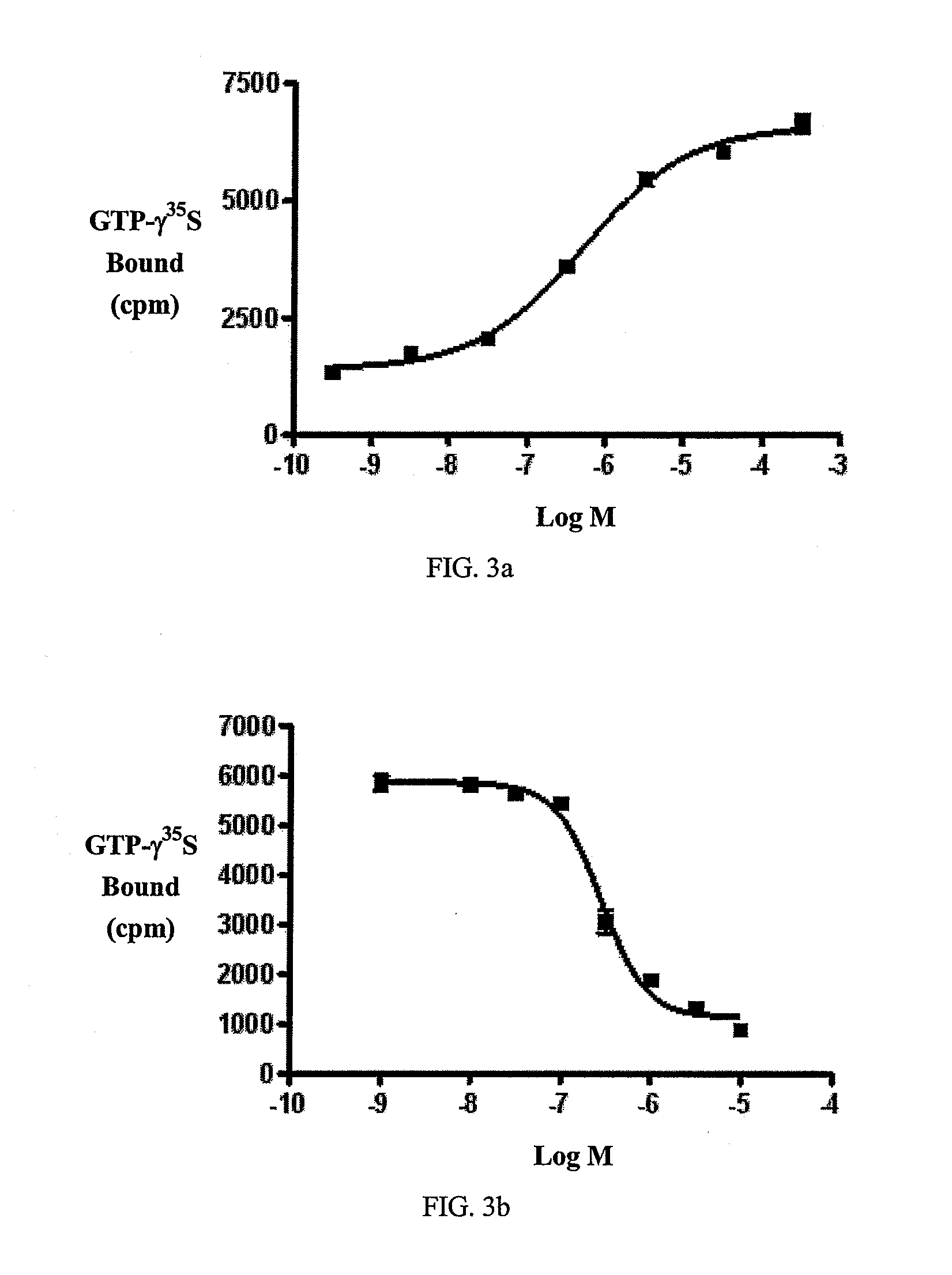
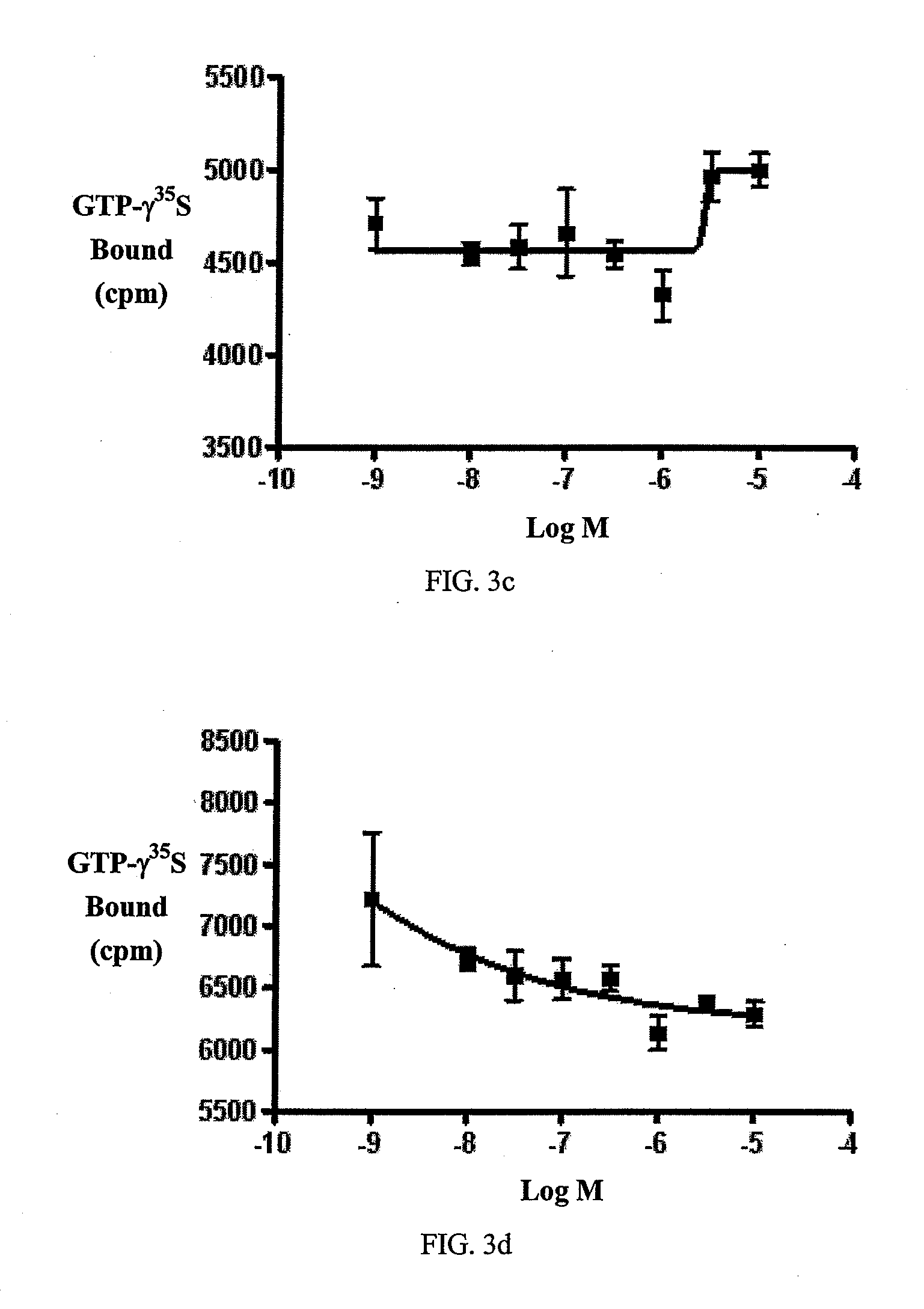
Beta-lactam cannabinoid receptor modulators
a technology of cannabinoid receptor and beta-lactam, which is applied in the direction of drug composition, immunological disorders, metabolism disorders, etc., can solve the problems of abdominal pain, vomiting and diarrhea, and affecting the absorption of beta-lactam, and achieves the effects of reducing the number of side effects, and improving the absorption ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1a
[0371](4(S)-phenyloxazolidin-2-on-3-yl)acetyl chloride. A solution of 1.0 equivalent of (4(S)-phenyloxazolidin-2-on-3-yl)acetic acid (Evans, U.S. Pat. No. 4,665,171) and 1.3 equivalent of oxalyl chloride in 200 mL dichloromethane was treated with a catalytic amount of anhydrous dimethylformamide (85 μL / milliequivalent of acetic acid derivative) resulting in vigorous gas evolution. After 45 minutes all gas evolution had ceased and the reaction mixture was concentrated under reduced pressure to give an off-white solid after drying for 2 h under vacuum.
example 1b
[0372](4(R)-phenyloxazolidin-2-on-3-yl)acetyl chloride. Prepared according to Example 1A, except that (4(R)-phenyloxazolidin-2-on-3-yl)acetic acid was used instead of (4(S)-phenyloxazolidin-2-on-3-yl)acetic acid (see, Evans & Sjogren, Tetrahedron Lett. 26:3783 (1985)).
example 1c
[0373]2-(4(S)-Phenyloxazolidin-2-on-3-yl)propanoyl chloride. A solution of 1 equivalent of Example 3A and 1.3 equivalent of oxalyl chloride in 200 mL CH2Cl2 (150 mL / g of propanoic acid derivative) was treated with a catalytic amount of anhydrous DMF (85 μL / mmole of propanoic acid derivative) resulting in vigorous gas evolution. After 45 min., all gas evolution had ceased and the reaction mixture was concentrated under reduced pressure to give an off-white solid after drying for 2 h. under vacuum.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap