Aptamer sandwich assays
a sandwich assay and aptamer technology, applied in the field of aptamer sandwich assays, can solve the problem of not having a method for readily identifying the two aptamers needed in sandwich type assays
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
SELEX with Insulin Receptor Proteins and ERBB2 Proteins
[0078]1.1 SELEX Process
[0079]The IR and ERBB2 receptor proteins were used as the target proteins for in vitro selection of aptamers from a pool of oligonucleotides (i.e., oligonucleotide library). The oligonucleotide library was composed of 70˜80-mer single stranded oligonucleotides containing 35˜45-mer random nucleotide regions of IR and ERBB2, and 17-mer fixed nucleotide regions at both 5′ end and 3′-end respectively. Specifically a synthetic oligonucleotide template containing 35˜45 random nucleotides flanked by fixed regions 5′-GAGTGACCGTCTGCCTG(SEQ ID NO: 58)-35˜45N-CAGCCACACCACCAGCC-3′(SEQ ID NO: 59) complementary to the primers 5′-GAGTGACCGTCTGCCTG-3′(SEQ ID NO: 58) and 5′-BB-GGCTGGTGGTGTGGCTG-3′(SEQ ID NO: 60), where BB denotes three biotin phosphoramidite couplings. This oligonucleotide library was used to identify aptamers with a relatively high binding affinity to IR or ERBB2 under simulated in vivo conditions, e.g., at
example 2
Identification of First Target Binding Aptamer
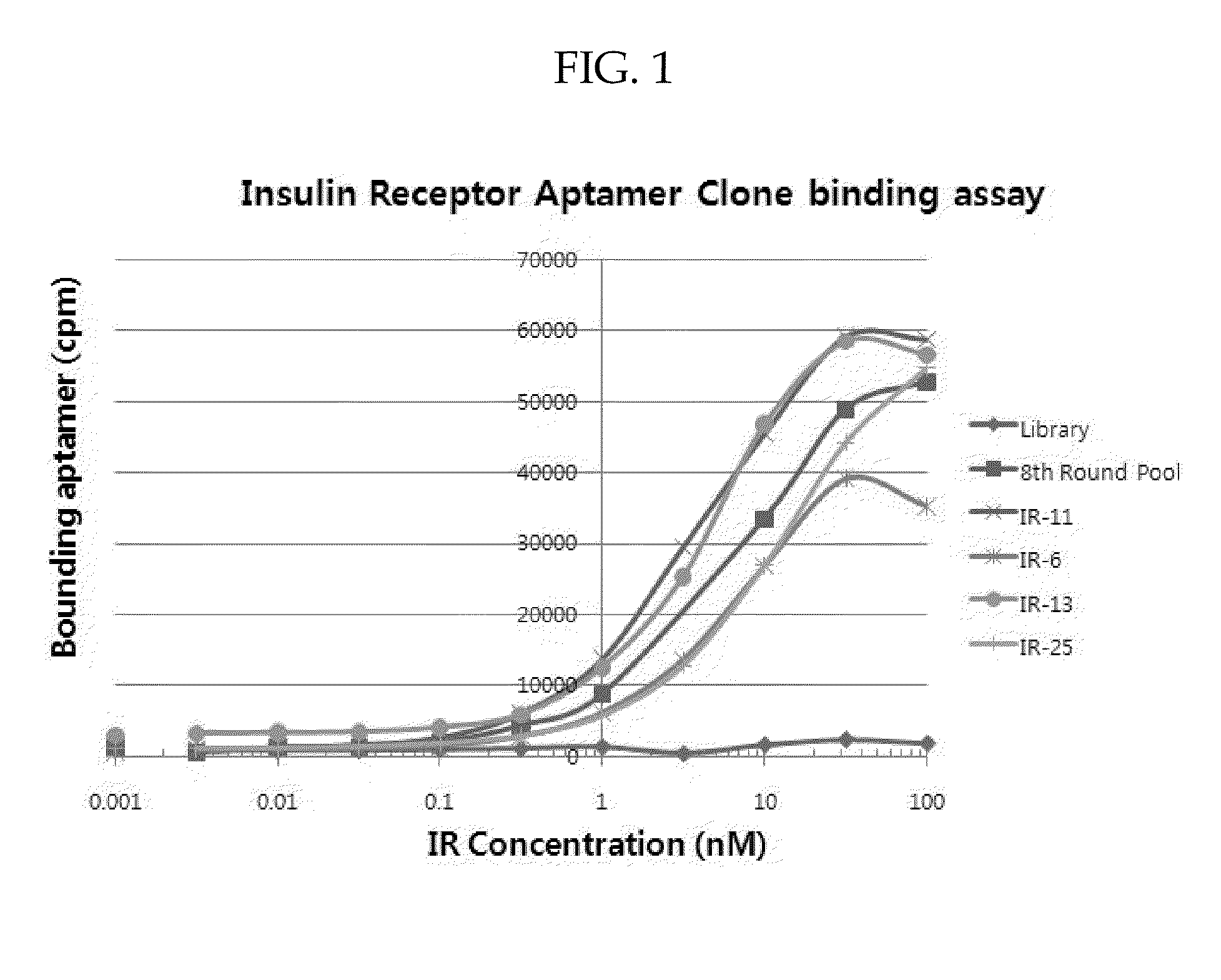
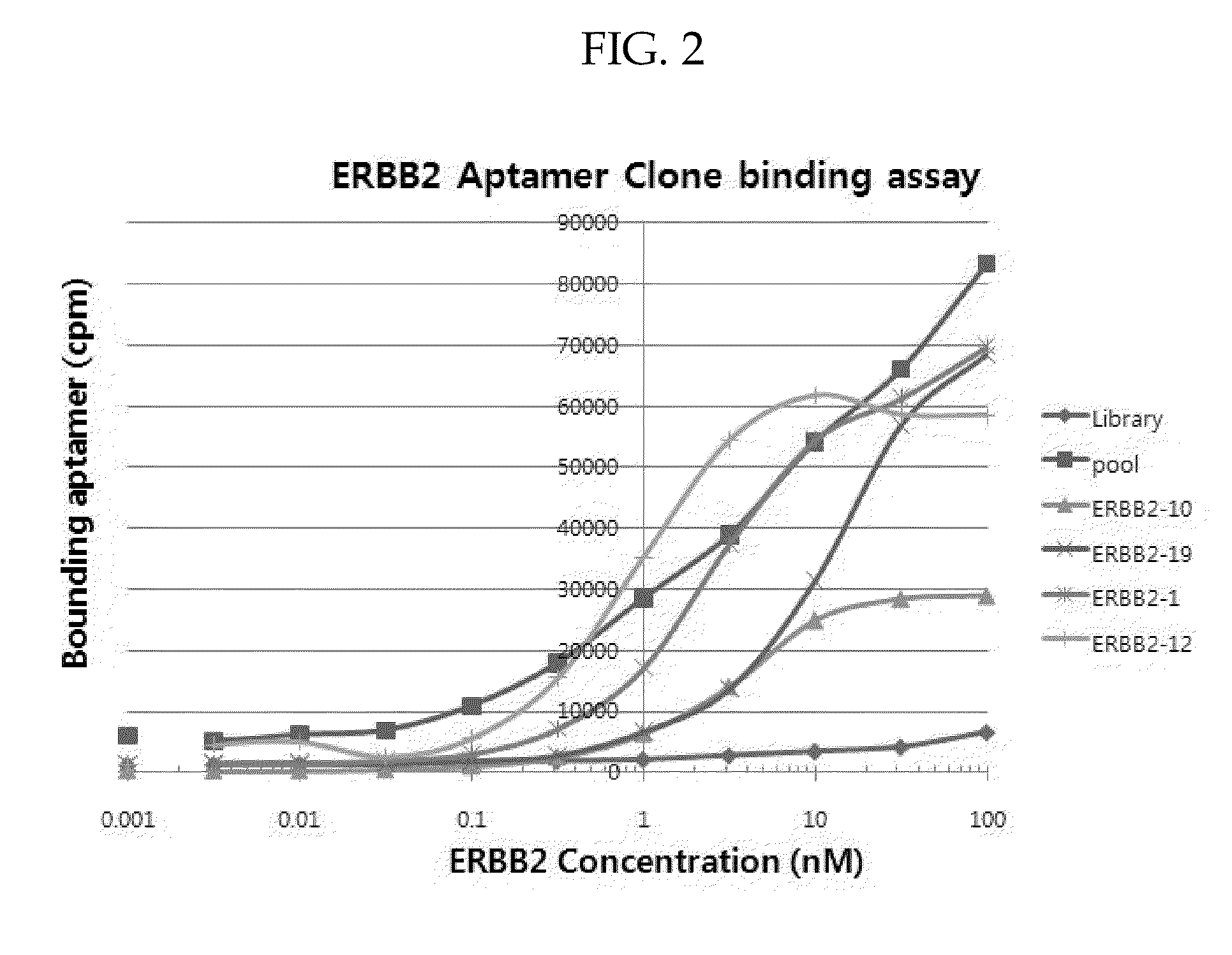
[0088]Aptamers identified using the above SELEX process were amplified by PCR and cloned as described above. Up to fifty randomly picked plasmid clones were sequenced. Several aptamers identified after eight rounds of SELEX process had sequences shown in Table 3 and Table 4. To verify that cloned aptamers bind to IR or ERBB2, aptamers that had same sequence in the aptamer pool were tested for binding affinity. Briefly, aptamers were incubated with various concentrations of target His-tagged proteins and the target His-tagged proteins were captured on Talon bead. Radio labeled aptamers that were bound to target proteins were then detected by phosphoimager.
[0089]Specifically, Aptamer-protein equilibrium dissociation constants (Kd's) were determined by the nitrocellulose-filter binding method as described by White et al. in Mol. Ther., 2001, 4(6), 567-73. For all binding assays, aptamers were dephosphorylated using alkaline phosphatase (New En
example 3
Identification of 2nd Target Binding Aptamers
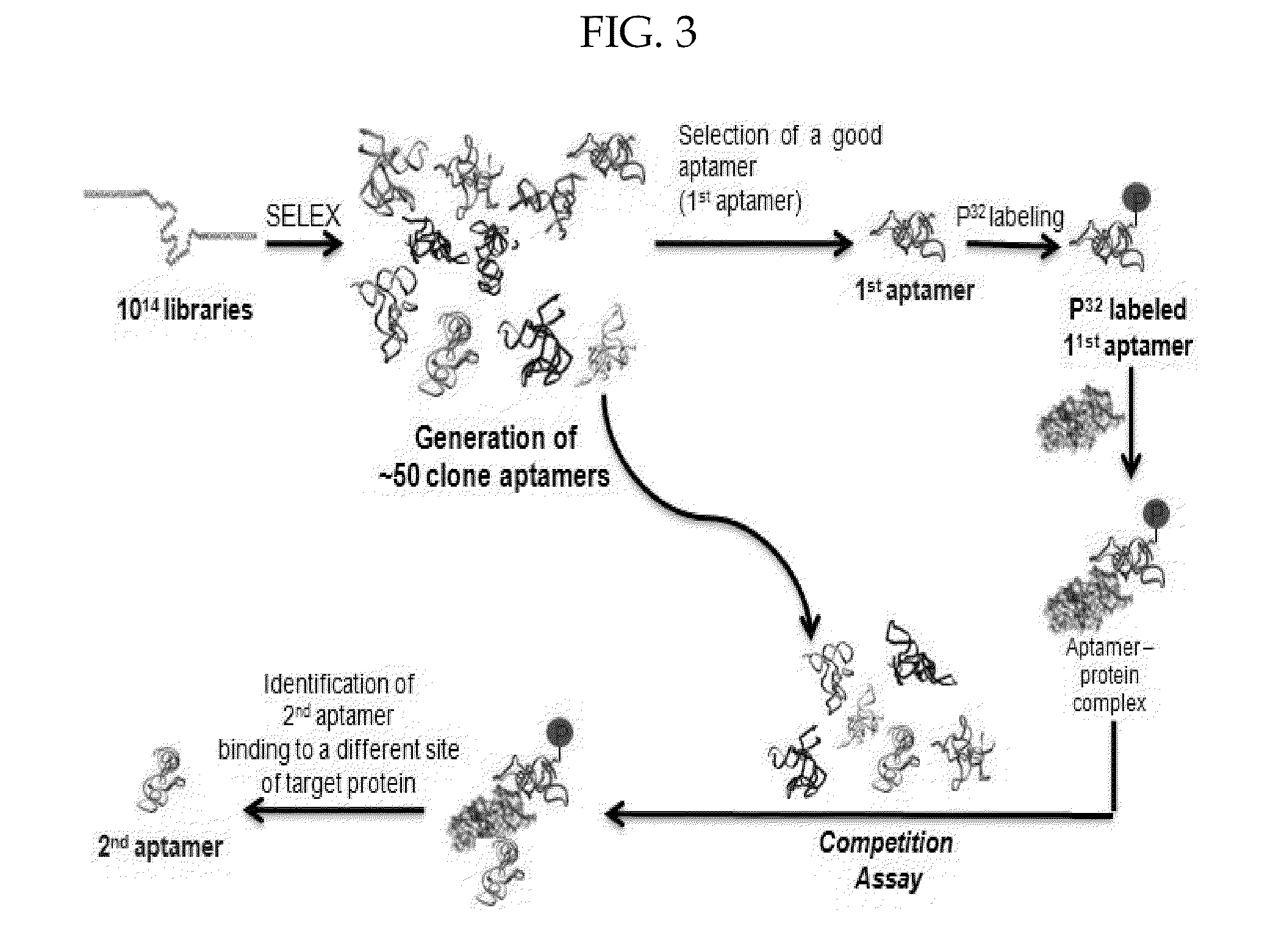
[0093]To identify aptamers that bind to a different site of the target protein compared to the 1st target binding aptamers identified above, a selection method using a competition assay was used. See the schematic illustration in FIG. 3.
[0094]Aptamers were labeled and allowed to refold as described above. See ‘example 2’ above. The 32P-labeled 1st target binding aptamer at a concentration of about 2 nM was preincubated with 10 nM of the target protein for 15 min at 37° C. to produce a labeled aptamer-protein complex (i.e., first target binding aptamer-protein complex). The resulting complex was incubated with other aptamers (at a concentration of about 30 nM) that were not labeled. It was expected that if the second aptamer shares the same binding site on the target protein with the 1st binding aptamer, there would be competitive binding between the labeled 1st target binding aptamer and the second target binding aptamer. If not, one would n
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap