Modified heterophasic polyolefin composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Example
Example 1
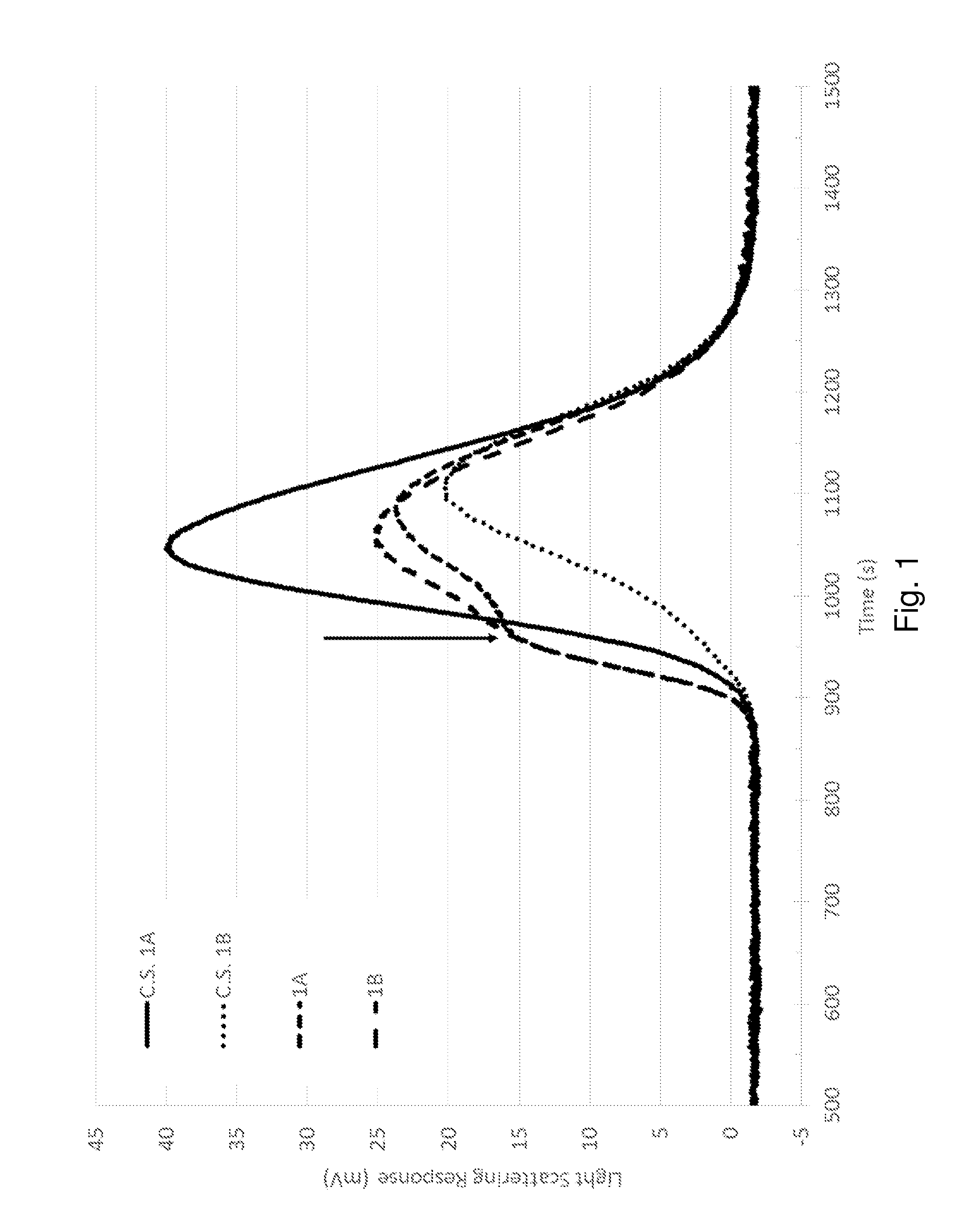
[0077]The following example demonstrates the modification of a heterophasic polyolefin composition and performance enhancements achieved, according to the method of the present invention.
[0078]Four heterophasic polymer compositions were produced. Comparative Sample 1A (C.S. 1A) was an unmodified polypropylene copolymer. Comparative Sample 1B (C.S. 1B) was made with the same polypropylene copolymer vis-broken using a peroxide. Samples 1A and 1B were made with the same vis-broken polypropylene copolymer compounded with 2-(furan-2-ylmethylene)malononitrile (Compound ID 1 from Table 7 below) as a compatibilizing agent. The general formulation for these samples is set forth in Table 1.
TABLE 1Heterophasic polypropylene copolymer formulationsComponentLoadingPolypropylene copolymerBalance(LyondellBasell Pro-Fax SD375S withapproximately 19% xylene solubles)Primary antioxidant (Irganox ® 1010)500 ppmSecondary antioxidant (Irgafos ® 168)1000 ppm Acid scavenger (calcium stearate)800 ppmPe
Example
Example 2
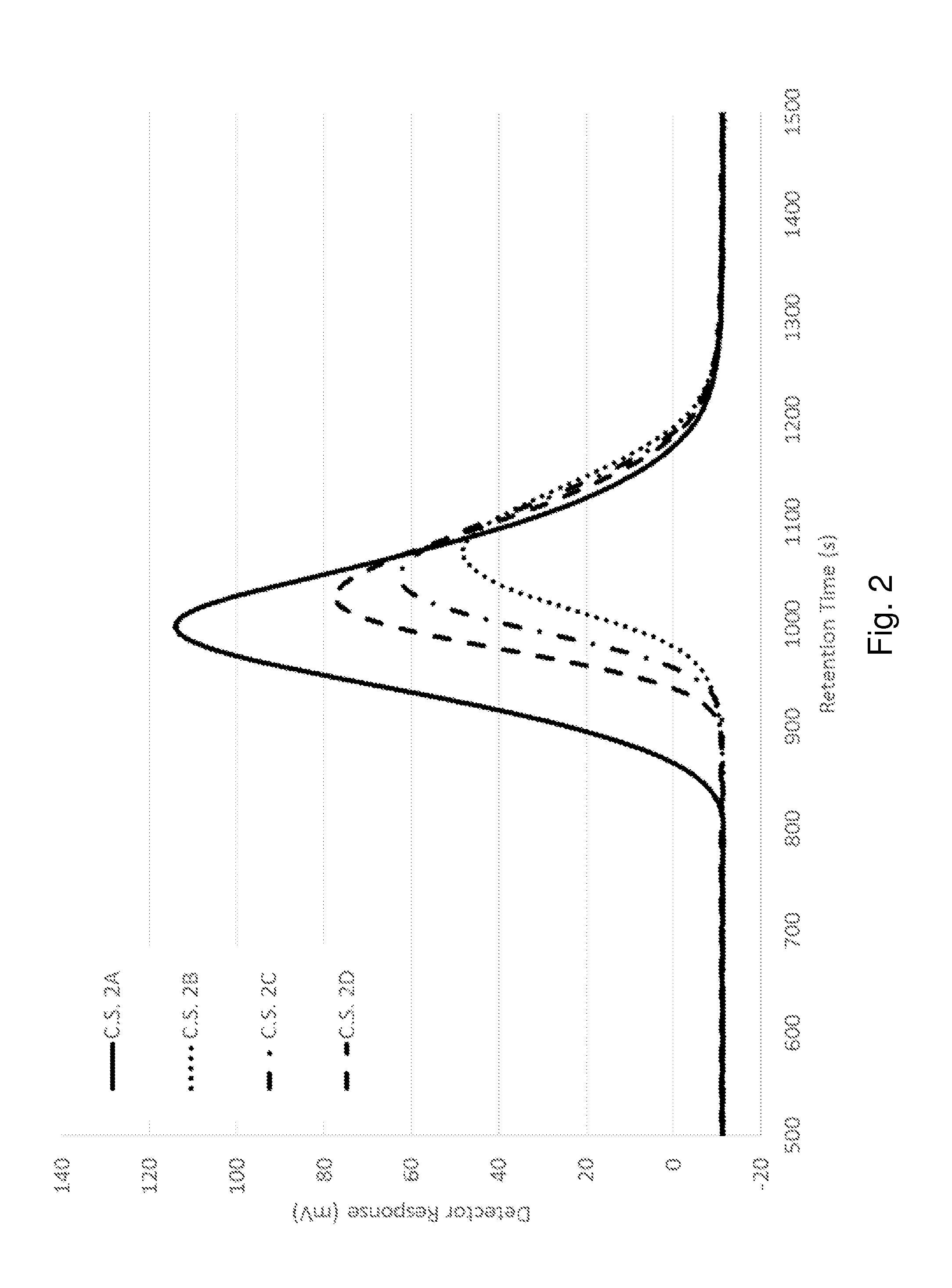
[0081]The following example investigates the effects of using a compatibilizing agent in a non-heterophasic polyolefin composition.
[0082]Four non-heterophasic polymer compositions were produced. Comparative Sample 2A (C.S. 2A) was an unmodified polypropylene polymer. Comparative Sample 2B (C.S. 2B) was made with the same polypropylene polymer vis-broken using a peroxide. Comparative Samples 2C and 2D were made with the same vis-broken polypropylene polymer compounded with 2-(furan-2-ylmethylene)malononitrile as a compatibilizing agent. The general formulation for these samples is set forth in Table 3.
TABLE 3Non-heterophasic polypropylene homopolymer formulationsComponentLoadingPolypropylene HomopolymerBalance(Total Petrochemicals 3276)Primary antioxidant (Irganox ® 1010)500 ppmSecondary antioxidant (Irgafos ® 168)1000 ppm Acid scavenger (calcium stearate)800 ppmPeroxide (Varox DBPH)See Table 4Additive (Compatibilizing Agent)See Table 42-(furan-2-ylmethylene)malononitrileIrgano
Example
Example 3
[0085]The following example demonstrates the production of several heterophasic polyolefin compositions as described above and investigates the performance enhancements achieved through the incorporation of the compatibilizing agents as described above.
[0086]In order to permit a comparison of the various compatibilizing agents and their effects on the physical properties of a heterophasic polymer composition, the relationship between melt flow rate and Izod impact of a commercially-available polypropylene copolymer (LyondellBasell Pro-Fax SD375S) was investigated by vis-breaking the polymer using several different loadings of a commercially-available peroxide (Varox BDPH). A compatibilizing agent according to the invention was not used in these compositions. The raw MFR and Izod impact values obtained from these measurements were then indexed to the MFR and Izod impact values of the virgin, unmodified polymer (not vis-broken) to provide relative values. The relative MFR and Iz
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap