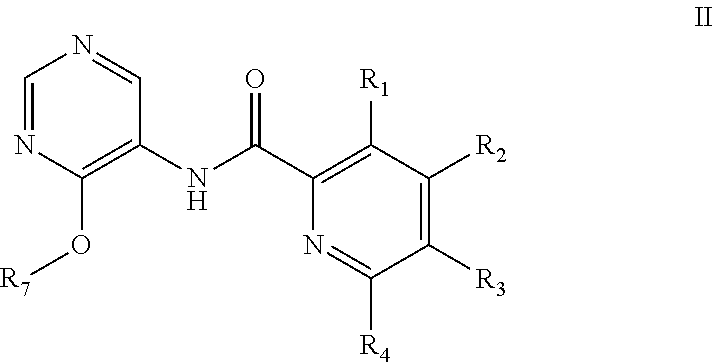
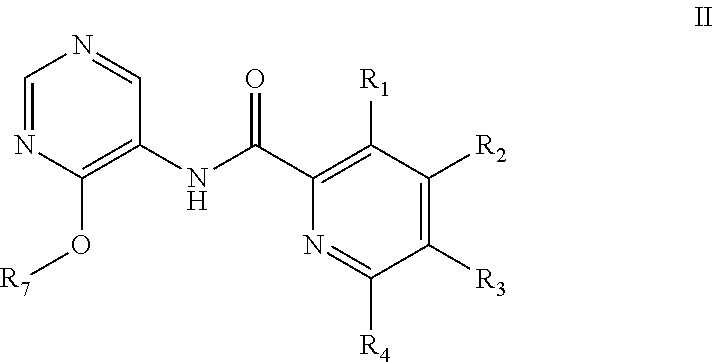
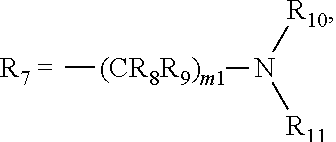Pyrimidine derivatives as cftr modulators
a technology of pyrimidine and derivatives, applied in the field of pyrimidine derivatives, can solve the problems of fluid imbalance, thick, sticky mucus and viscous secretions to accumulate, and achieve the effect of reducing the number of cftr and cftr, and improving the stability of cftr
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 6-(2,6-difluorophenyl)-5-fluoro-N-(4-(piperidin-4-ylmethoxy)pyrimidin-5-yl)picolinamide hydrochloride (1)
[0056]
(1) tert-butyl 4-(((5-aminopyrimidin-4-yl)oxy)methyl)piperidine-1-carboxylate (C1)
[0057]
[0058]At room temperature, NaH (67 mg, 2.79 mmol) was added to a solution of tert-butyl 4-(hydroxymethyl)piperidine-1-carboxylate (B1) (500 mg, 2.32 mmol) in THF (tetrahydrofuran) (10 mL) and stirred for 1 hour. 4-chloropyrimidin-5-amine (A) (346 mg, 2.67 mmol) was then added. The reaction mixture was then heated to 100° C. under nitrogen and stirred for 4 hours, cooled to room temperature (20-30° C.) and concentrated in vacuo. The residue was purified with flash column (eluent: 10-30% ethyl acetate / petroleum ether) to obtain the product C1 (308 mg, 1.0 mmol).
(2) Synthesis of tert-butyl 4-(((5-(6-(2,6-difluorophenyl)-5-fluoropicolinamido)pyrimidin-4-yl)oxy)methyl)piperidine-1-carboxylate (E1)
[0059]
[0060]Compound (C1) (49 mg, 0.16 mmol), 6-(2,6-difluorophenyl)-5-fluoropicolini
example 2
Synthesis of N-(4-(azetidin-3-ylmethoxy)pyrimidin-5-yl)-6-(2,6-difluorophenyl)-5-fluoropicolinamide hydrochloride (2)
[0063]
[0064]Following the procedure described in Example 1, and substituting compound B1 in Step (1) with tert-butyl 3-(hydroxymethyl)azetidine-1-carboxylate (B2), the title compound 2 was obtained.
example 3
Synthesis of 6-(2,6-difluorophenyl)-5-fluoro-N-(4-(piperidin-4-yloxy)pyrimidin-5-yl)picolinamide hydrochloride (3)
[0065]
[0066]Following the procedure described in Example 1, and substituting compound B1 in Step (1) with tert-butyl 4-hydroxypiperidine-1-carboxylate (B3), the title compound 3 was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap