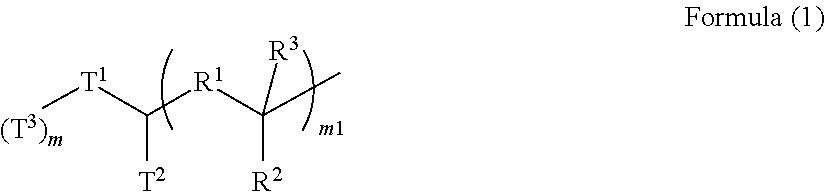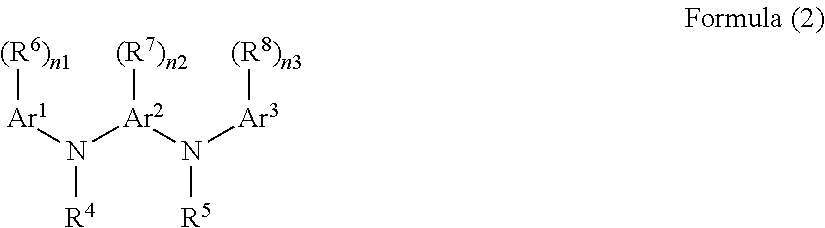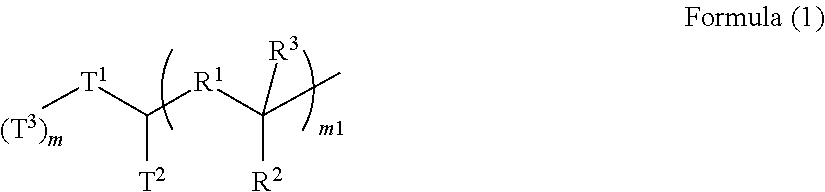Resist underlayer film-forming composition that contains triaryldiamine-containing novolac resin to which aromatic vinyl compound is added
a technology of aromatic vinyl compound and film-forming composition, which is applied in the direction of instruments, photomechanical devices, coatings, etc., to achieve the effect of reducing the glass transition temperature (tg), and improving the thermal reflow property of the polymer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0158]A 300-mL four-necked flask was charged with 41.98 g (0.161 mol) of N,N′-diphenyl-1,4-phenylenediamine (available from Tokyo Chemical Industry Co., Ltd.), 31.02 g (0.161 mol) of 4-amyloxybenzaldehyde (available from Tokyo Chemical Industry Co., Ltd.), 94.75 g (0.537 mol) of 4-(tert-butoxy)styrene (available from Wako Pure Chemical Industries, Ltd.), and 172.37 g of propylene glycol monomethyl ether (available from Kanto Chemical Co., Inc.). To the mixture was added 4.65 g (0.048 mol) of methanesulfonic acid (available from Tokyo Chemical Industry Co., Ltd.), and the resultant mixture was stirred and heated to 135° C. for dissolution and initiation of polymerization. After the elapse of 18 hours, the mixture was left to cool to room temperature, and then a solvent mixture of 1,000 g of methanol (available from Kanto Chemical Co., Inc.), 1,000 g of ultrapure water, and 100 g of 30% aqueous ammonia (available from Kanto Chemical Co., Inc.) was added to the mixture for reprecipitation
example 2
[0161]1.00 g of pDPPDA-AOBA-TBOS as a polymer, 0.20 g of 2,2-bis[3,5-bis[(2-methoxy-1-methylethoxy)methyl]-4-hydroxyphenyl]propane (compound prepared by dehydration and condensation of four methylol groups of Formula (4-20) with propylene glycol monomethyl ether, abbreviated as “PGME-BIP-A” and shown by Formula (5-1)) serving as a crosslinking agent, 0.020 g of pyridinium-p-phenolsulfonate serving as a crosslinking catalyst (acid catalyst), and 0.001 g of a surfactant (product name: MEGAFAC [trade name] R-30N, available from DIC Corporation, fluorine-containing surfactant) were dissolved in 7.00 g of propylene glycol monomethyl ether and 16.36 g of propylene glycol monomethyl ether acetate, to thereby prepare a resist underlayer film-forming composition.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap