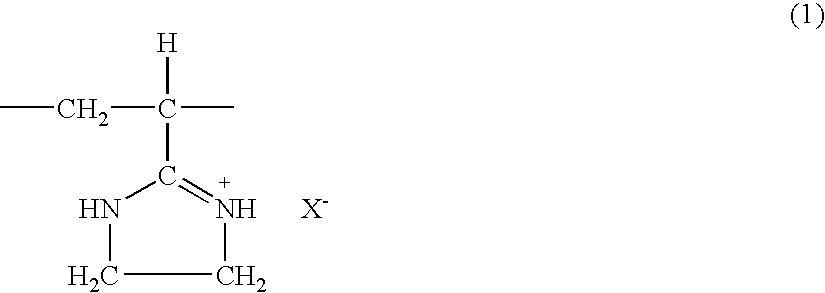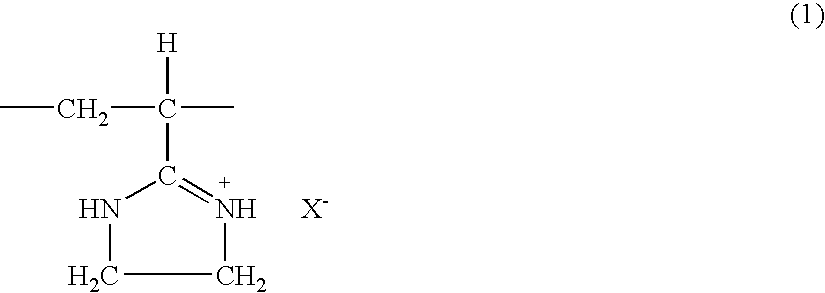Branched polyvinyl imidazoline acid salt, polymeric flocculant comprising same, and process for preparing same
a polymer flocculant and polyvinyl imidazoline technology, which is applied in the field of polymeric flocculant comprising the process of preparing the branched polyvinyl imidazoline acid salt, can solve the problems of increasing the volume of dehydrated cakes, increasing the cost of treatment, and increasing environmental problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
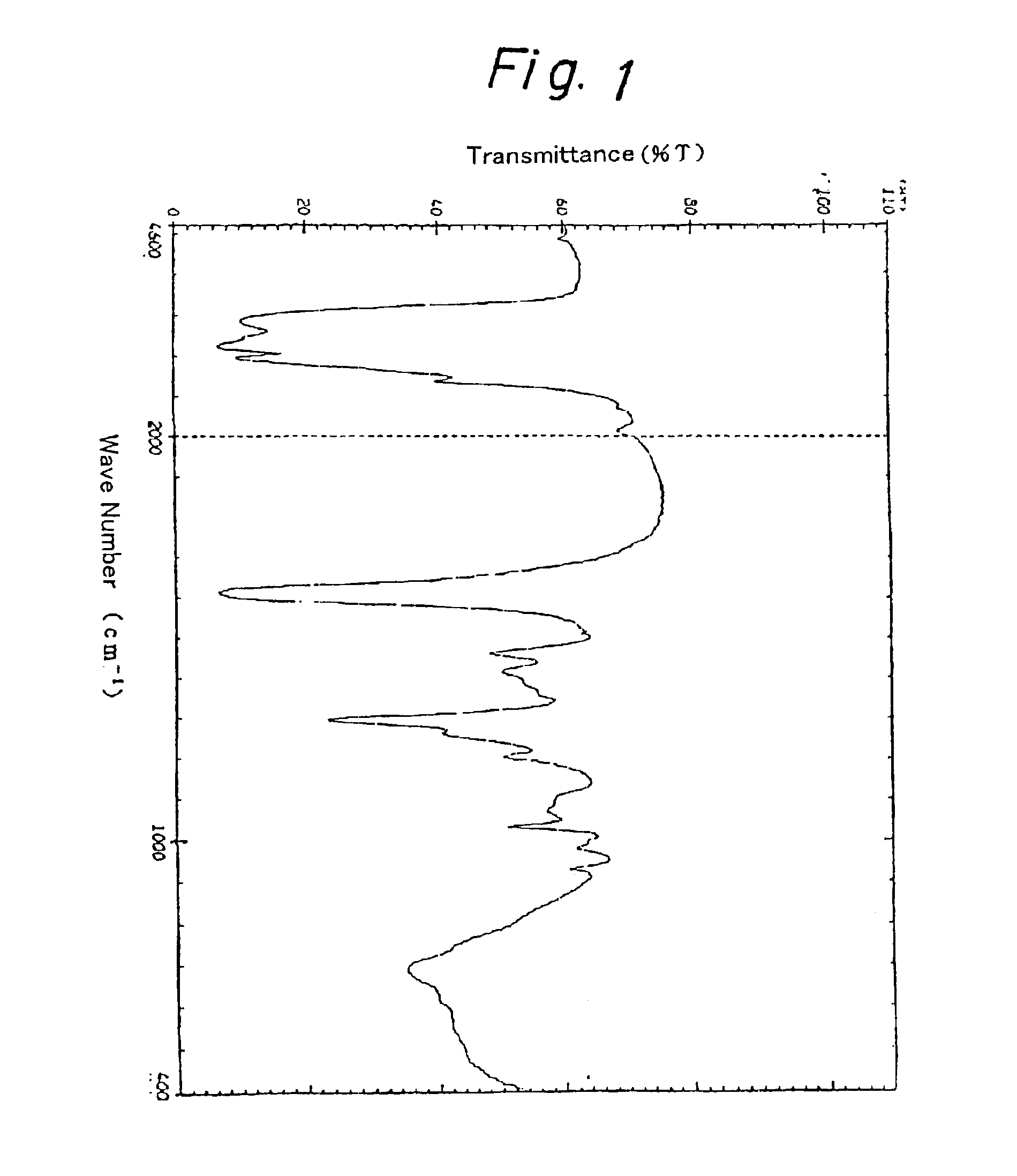
[0044]A flask was charged with 10.0 g of the polyacrylonitrile obtained in Starting Material Production Example 1, 100.0 g of ethylenediamine, 12.0 g of ammonium chloride, and 80.0 g of 1-butanol. After purging with nitrogen, the internal temperature was raised to 95° C. with stirring. The system was held at 95° C. for 4.5 hours with stirring, and then cooled. The viscous reaction mixture was taken out, poured into acetone, and washed several times with acetone. The washed mixture was dried under reduced pressure to obtain 22.9 g of a white solid. The resulting solid (5.0 g) was added to 300 ml of a methyl alcohol solution containing 10% by weight of hydrochloric acid. The system was mixed with stirring for 1 hour at room temperature. The mixture was poured into acetone, and washed several times with acetone. The washed mixture was dried under reduced pressure to obtain 4.5 g of a white solid. The infrared absorption spectrum of the resulting solid is shown in FIG. 1. Based on its infr
example 2
[0047]The same procedure as in Example 1 was performed, except that 10.0 g of the polyacrylonitrile obtained in Starting Material Production Example 2 was used instead of the polyacrylonitrile obtained in Starting Material Production Example 1. As a result, 19.1 g of a white solid was obtained. The resulting solid (5.0 g) was added to 300 ml of a methyl alcohol solution containing 10% by weight of hydrochloric acid. Then, the same procedure as in Example 1 was performed to obtain 4.8 g of a white solid. Its infrared absorption spectrum and chlorine content showed the resulting solid to be a polyvinyl imidazoline hydrochloride of the aforementioned chemical formula (1). The polyvinyl imidazoline hydrochloride had a Huggins' constant k′ of 0.25 and an intrinsic viscosity [η] of 4.4 based on measurements at 30° C. in a 0.1 mol / l aqueous solution of sodium chloride.
[0048]An aqueous solution (0.3% by weight) of the polyvinyl imidazoline hydrochloride was prepared, and subjected to perform
example 3
[0049]The same procedure as in Example 1 was performed, except that 10.0 g of the polyacrylonitrile obtained in Starting Material Production Example 3 was used instead of the polyacrylonitrile obtained in Starting Material Production Example 1. As a result, 20.6 g of a white solid was obtained. The resulting solid (5.0 g) was added to 300 ml of a methyl alcohol solution containing 10% by weight of hydrochloric acid. Then, the same procedure as in Example 1 was performed to obtain 4.6 g of a white solid. Its infrared absorption spectrum and chlorine content showed the resulting solid to be a polyvinyl imidazoline hydrochloride of the aforementioned chemical formula (1). The polyvinyl imidazoline hydrochloride had a Huggins' constant k′ of 0.50 and an intrinsic viscosity [η] of 6.9 based on measurements at 30° C. in a 0.1 mol / l aqueous solution of sodium chloride.
[0050]An aqueous solution (0.3% by weight) of the polyvinyl imidazoline hydrochloride was prepared, and subjected to perform
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap