Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1 results about "Attenuated vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An attenuated vaccine is a vaccine created by reducing the virulence of a pathogen, but still keeping it viable (or "live"). Attenuation takes an infectious agent and alters it so that it becomes harmless or less virulent. These vaccines contrast to those produced by "killing" the virus (inactivated vaccine).
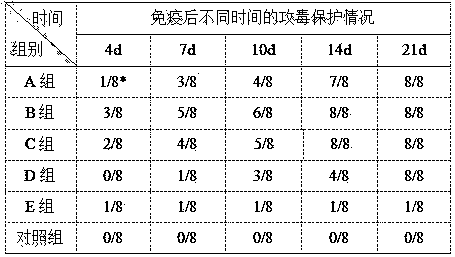
Immunopotentiator and vaccine preparation for emergency vaccination of Newcastle disease
InactiveCN104069480AProduced fastEasy to operateViral antigen ingredientsPeptide/protein ingredientsBiotechnologyHemagglutinin
Owner:GUANGZHOU SOUTH CHINA BIOLOGICAL MEDICINE
Popular searches
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap