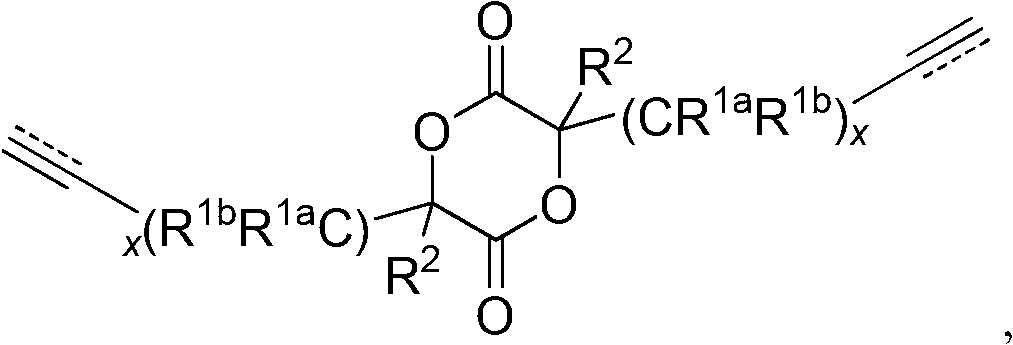
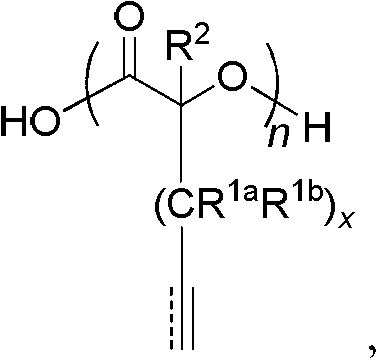
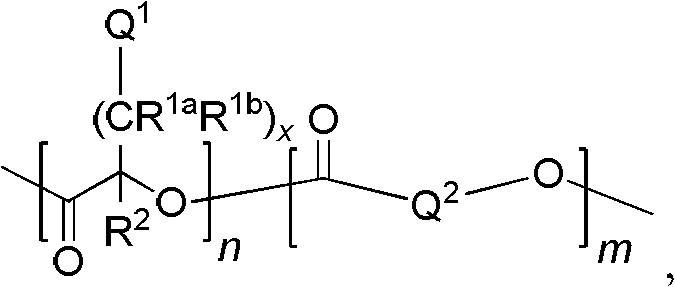
Process for preparing cyclic esters comprising unsaturated functional groups and polyesters prepared from same
A technology for cyclic esters and copolymers, applied in the field of preparing cyclic esters containing unsaturated functional groups and polyesters prepared therefrom, can solve problems such as suboptimal, large solvent yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] Embodiment 1 (hypothetical embodiment)
[0131]Under high vacuum (~0.02 Torr), α-hydroxy acid, 2-hydroxy-4-pentenoic acid (50 g) was polymerized in bulk at 120 °C using 150 μl (0.95 mol%, mol) of sulfuric acid as a polymerization catalyst and last for 2 hours. Zinc oxide, ZnO (1 g) was added as a transesterification catalyst and the desired monomer was distilled off under high vacuum (~0.02 Torr) and high temperature (230°C to 240°C) and collected on a cold trap (on dry ice superior). Optionally, the collected monomer is purified by recrystallization, for example by dissolving into warm diethyl ether followed by cooling on dry ice overnight to recrystallize the monomer).
Embodiment 2
[0133] 0.20 g (1.02 mmol) of diallyl lactide and 1.30 g (9.03 mmol) of lactide were added to the dry flask under a nitrogen atmosphere. The flask was immersed in an oil bath heated at 130°C. After the solid had melted, 15.4 μl (0.20 mmol) of methyl glycolate and 43.8 μl of a 0.247M solution of tin 2-ethylhexanoate in toluene (containing 0.0108 mmol of tin 2-ethylhexanoate) were added. The liquid was stirred at 130°C for 4 hours. After the liquid had cooled to room temperature, the liquid was dissolved in chloroform and precipitated into cold methanol. The resulting precipitate was collected and dried overnight under vacuum at 45°C-50°C. 1.10 g of polymer were obtained, yield: 73.3%. 1 H NMR (in CDCl 3 Medium, 300MHz): 5.7ppm-5.9ppm (b, CH 2 =CH-),5.1ppm-5.3ppm(b,CH 2 =CH-, O=C-CH-O-), 3.75ppm (b, due to the initiator methyl glycolate, CH 3 O-C=O),2.6ppm-2.8ppm(b,CH 2 =CH-CH 2 -), 1.4ppm-1.7ppm (b, CH 3 -).
[0134] The molecular weight by NMR was 13,400 Daltons. The c
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap