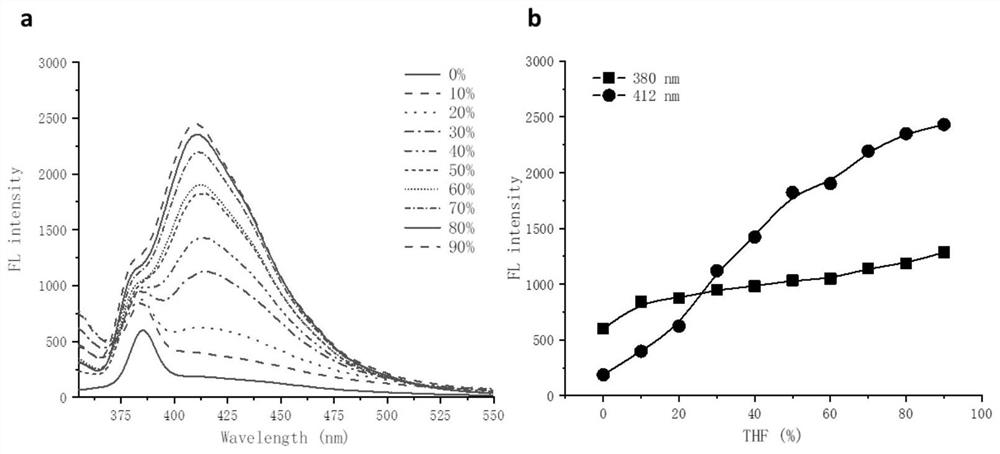
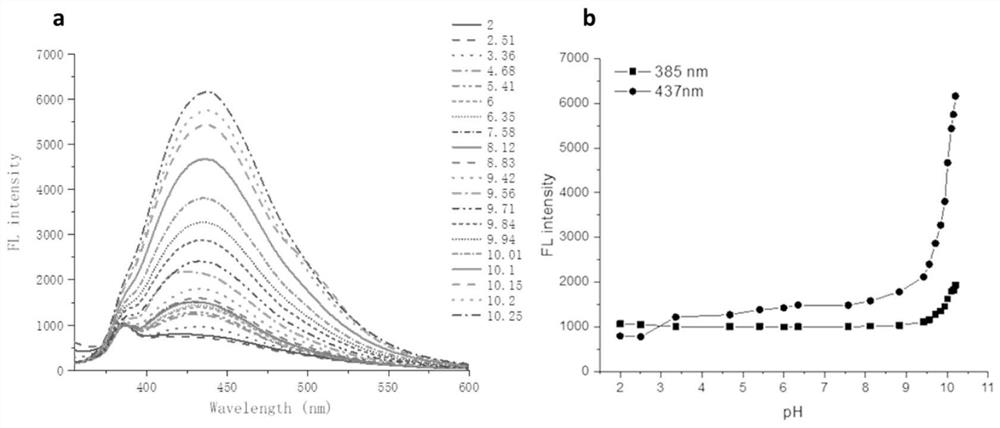
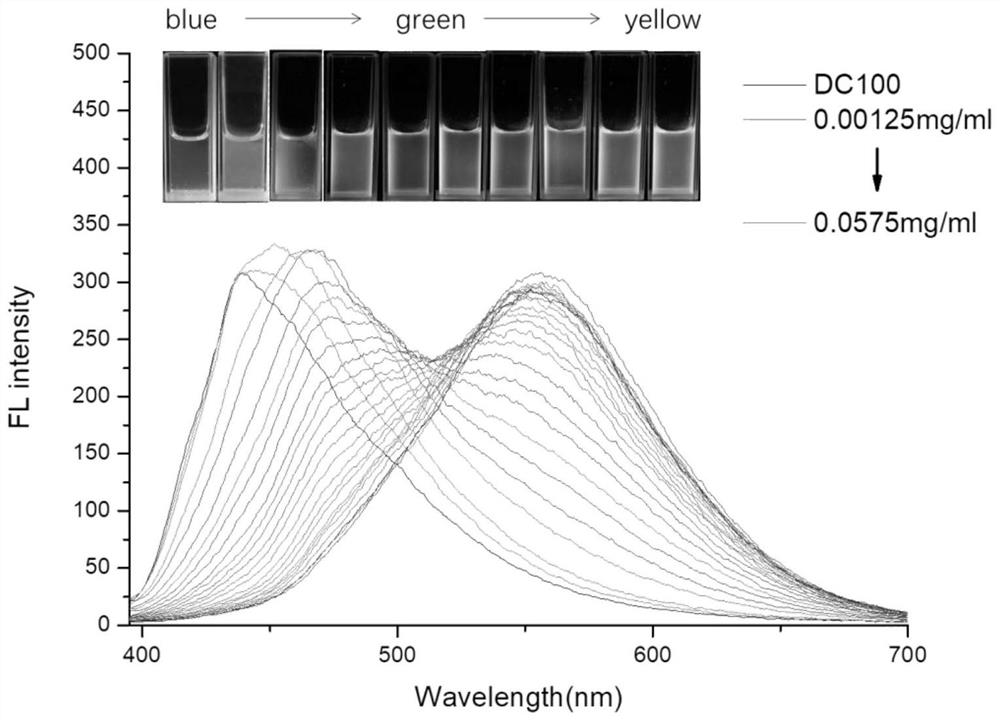
Multifunctional polyurea acid derivative as well as preparation method and application thereof
A technology of derivatives and ureidic acid, applied in the application of fluorescent probes and drug delivery systems, multifunctional polyureidic acid derivatives and its preparation field, can solve the problem of unreported pseudo polyamino acids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1: Synthesis of Polyurea Acid Homopolymer (DL)
[0078] This embodiment provides a two-component polyurea acid homopolymer (DL) with different number of segments, which is mainly produced by solution polymerization of an isocyanate component (component B) and an amino component (component A); wherein, The isocyanate component requires that the average functional group in the polyisocyanate monomer is 2, that is, the diisocyanate monomer is lysine diisocyanate; the amino component is lysine ethyl ester dihydrochloride.
[0079] The specific preparation steps are as follows:
[0080] 1) Prepare the raw materials according to the formula, take by weighing lysine ethyl ester dihydrochloride (4.092g) three-pump tee, and add dimethylformamide (120ml), triethylamine ( 7.2~20ml) for premixing, the speed is controlled at 200~500r / rev, and the constant stirring speed is maintained at room temperature and normal pressure until it is completely dissolved;
[0081] 2) take
Embodiment 2
[0085] Example 2: Synthesis of Polyurea Acid Homopolymer (DC)
[0086] This embodiment provides a one-component polyurea-based homopolymer (DC), which is mainly produced from an amino component (component A) by a phosgene method; wherein, the amino component is cystine.
[0087] 1) Prepare the raw materials according to the formula, weigh cystine (14.418g) and add it to the reaction flask, pump three connections, add dichloromethane (60ml) under inert gas atmosphere for premixing, reduce the temperature to 0°C, Keep a constant stirring speed under normal pressure until it is completely dissolved;
[0088] 2) Weigh triphosgene (5.927g), dissolve in dichloromethane;
[0089] 3) Under an inert gas atmosphere, add triphosgene dropwise to the reaction flask at 0°C to react with A for 15min, and then react at room temperature for 8h;
[0090] 4) After the reaction is finished, the product is poured into a Buchner funnel for suction filtration, and the dichloromethane and water therei
Embodiment 3
[0091] Example 3: Synthesis of Polyurea Acid Alternating Copolymer (DLL)
[0092] This embodiment provides a two-component polyurea-based alternating copolymer (DLL), which is mainly produced by solution polymerization of an isocyanate component (component B) and an amino component (component A); wherein, the isocyanate component lysine diisocyanate was selected for the sub-component; lysine was selected for the amino component.
[0093] The specific preparation steps are as follows:
[0094] 1) Prepare the raw materials according to the formula, weigh lysine (2.422g) with three-pump tee, add dimethylformamide (120ml) to the reaction flask under inert gas atmosphere for premixing, and control the rotation speed at 200~500r / Rotate, keep constant stirring speed at room temperature and normal pressure until completely dissolved;
[0095] 2) take by weighing lysine diisocyanate (5.587g), ensure that the molar feeding ratio of component A and component B is 1:1, dilute with pre-d
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap