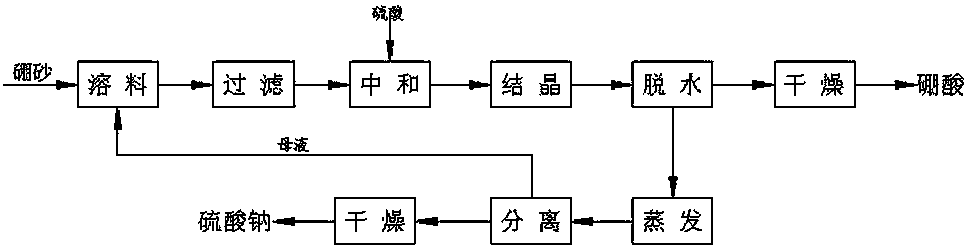
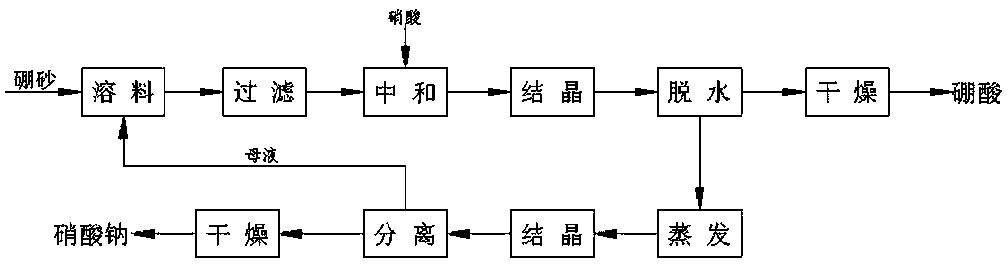
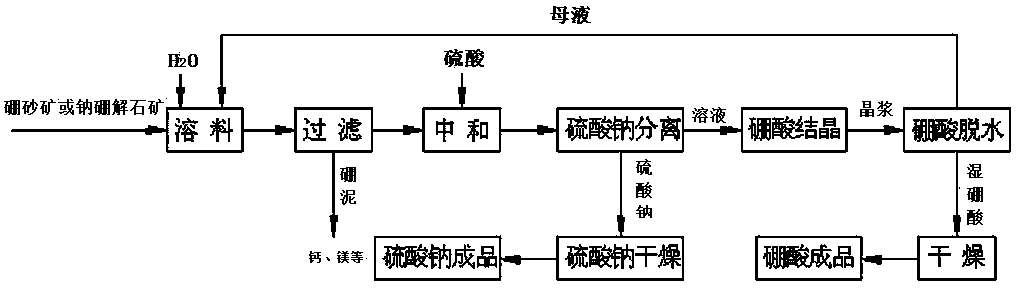
Method for preparing boric acid and sodium nitrate by treating ulexite (kramerite) or ascharite with nitric acid
A technology of boron-magnesium ore and sodium nitrate, which is applied in the preparation of alkali metal nitrate, magnesium carbonate, boron oxide compounds, etc., can solve the problem of failure to propose a method for preparing sodium nitrate and calcium and magnesium compounds, high price of sodium hydroxide, control problems such as unfavorable costs, to achieve the effect of improving the level of comprehensive utilization, increasing economic benefits, and controlling the impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Such as Figure 4 Shown, the present invention prepares the method for boric acid and sodium nitrate with nitric acid treatment sodium borolysis (calcium) stone ore or boron magnesium ore, prepares according to the following steps:
[0039] 1000kg sodium borolysis (calcium) stone (containing B 2 o 3 35%, Na 2 O 4.91%, CaO 14.1%, MgO 0.47%) ore is mixed with water and washed to remove chloride in the raw ore. Then mix the washed raw ore and 5000L boric acid mother liquor (co-saturated solution of boric acid and sodium nitrate at 25°C) in an acidolysis reactor, heat to 60°C under stirring conditions, and then add nitric acid with a concentration of 68% 473Kg, the temperature was raised to 85°C, and the reaction was timed and kept for 60 minutes. When the pH value at the end of the reaction was stable at 2.5-3.5, the reaction ended. Then pump the slurry into a filter press for filtration to obtain acid hydrolysis solution and a small amount of boron mud. The acid soluti
Embodiment 2
[0043] Such as Figure 4 Shown, the present invention prepares the method for boric acid and sodium nitrate with nitric acid treatment sodium borolysis (calcium) quarry, prepares according to the following steps:
[0044] 1000kg boron magnesium ore (containing B 2 o 3 38%, CaO 1.8%, MgO18%) ore is crushed to 40 mesh, mixed with circulating mother liquor (water for the first time) in the acid hydrolysis reactor, heated to 75°C under stirring conditions, and then added with 68% nitric acid 887.1Kg, then raise the temperature to 95°C, time the heat preservation reaction for 60 minutes, control the pH value at the end of the reaction to be stable at 2.5-4.5, and the reaction is over. Then pump the slurry into a filter press for filtration to obtain acid hydrolysis solution and a small amount of boron mud. The acid solution is sent to the cooling crystallizer, and the boric acid crystals are precipitated by cooling, and the boric acid is separated, washed, and dried to obtain indu
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap