Preparation method for novel adjuvant piglet Escherichia coli inactivated vaccine
A technology of Escherichia coli and inactivated vaccine, which is applied to medical preparations containing active ingredients, antibacterial drugs, pharmaceutical formulations, etc., can solve the problems of large residual amount, poor immune effect, and unfavorable use of meaty poultry, etc. Avoid immune tolerance, eliminate application, reduce the effect of animal disturbance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0028] Example 1
[0029] Escherichia coli strains: isolated, identified, screened and conserved by the research team of the Highland Animal Disease Detection Center, College of Animal Science, College of Agriculture and Animal Husbandry, Tibet University.
[0030] Mannitol 2013103101, Chengdu Kelong Chemical; LB medium: Tryptone10g, YeastExtract5g, NaCl10g, with ddH 2 Dilute the volume to 1000ml with O, adjust the pH to 7.0, sterilize by autoclaving, and store at 4°C.
[0031] LB medium plate: Tryptone 10g, YeastExtract 5g, NaCl 10g, agar 20g, fixed to ddH 2 O1000ml, adjust pH to 7.0, autoclave for 20min, pour the dissolved LB medium into a sterilized plate under an alcohol lamp, cool and solidify and store at 4°C for later use.
[0032] Kanamycin (stock solution): Kanamycin 0.3g, dilute to ddH 2 O10ml, filter and sterilize with 0.22um filter, and store at -20℃ for later use.
[0033] Chloramphenicol (storage solution): 0.34g of chloramphenicol, dilute to 10ml of absolute et
Example Embodiment
[0074] Example 2
[0075] Immunization method:
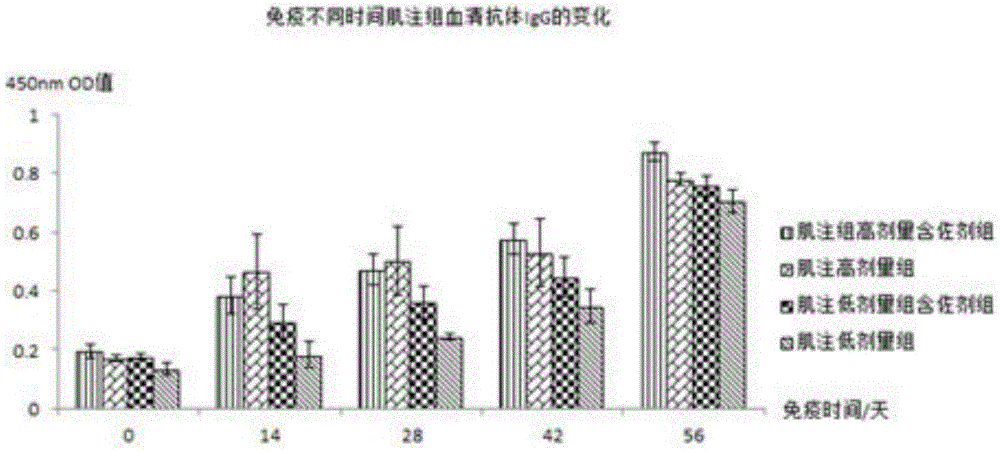
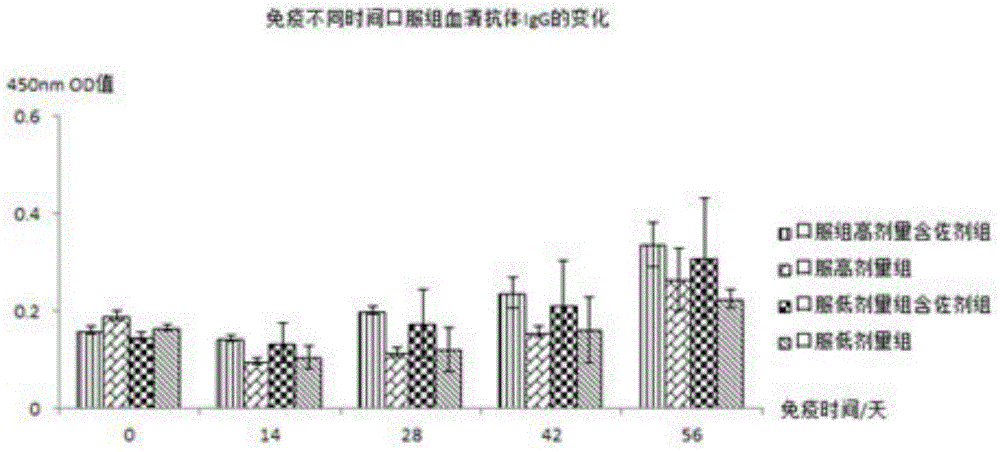
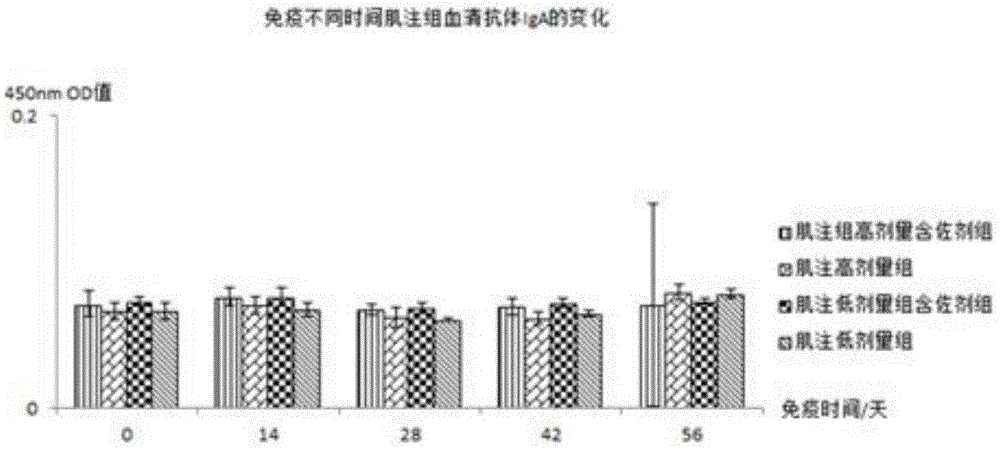
[0076] The piglets were orally immunized with Escherichia coli inactivated adjuvant vaccine according to the immunization schedule of 0, 14, 28, and 42 days for a total of 4 times. Before each immunization, the vaccine was reconstituted with distilled water and injected intramuscularly: 4ul-40ul per gram of animal body weight The amount of bacteria is 10 6 -10 10 CFU / ml vaccine; oral 1ml-10ml bacteria per kg animal body weight is 10 6 -10 10 CFU / ml vaccine, stirred until the temperature is lower than room temperature and fed to the animals to be immunized.
Example Embodiment
[0077] Example 3
[0078] (1) Detection of immune effect
[0079] Immunization: Mice were immunized with adjuvanted and unadjuvanted vaccines respectively, and the immunization schedule was performed on 0, 14, 28, and 42 days. Intramuscular injection or oral immunization was used for a total of 4 times. The vaccine was reconstituted with distilled water before each immunization. Immunization by intramuscular injection: 4ul-40ul per gram of animal body weight, and the bacterial volume was 10 6 -10 10 CFU / ml vaccine; Oral immunization: 1-10ml bacterial volume is 10 6 -10 10 The vaccine at CFU / ml was stirred until the temperature was lower than room temperature and fed to the animals to be immunized.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap