Preparation and analytical application of Rosamine fluorescence probe for detecting H2S
A fluorescent probe, separation and purification technology, applied in the field of organic small molecule fluorescent probe, to achieve the effect of simple synthesis process, simple method and strong anti-interference ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Preparation of nitrogenated rhodamine Ros-N 3
[0027] Weigh 2-3g of 3-(diethylamine)phenol and 1-2g of p-nitrobenzaldehyde and dissolve it in 30-50 propionic acid, add 0.1-0.2g of p-toluenesulfonic acid, and reflux under stirring at 65°C 16 hours. After the reaction, the mixture was cooled to room temperature, added to 3mol / L sodium acetate solution, the suspension was extracted with dichloromethane solution, the organic phases were combined, and propionic acid was removed by back extraction with water. Dry over anhydrous sodium sulfate and concentrate. The obtained solid was dissolved in a mixture of methanol and dichloromethane (1:1). Add 1-1.5 g of chloro-p-benzoquinone, stir rapidly for 2 hours, and concentrate in vacuo. Gradient column purification (dichloromethane: methanol = 50:1-9:1, v / v). The purple nitrorosamine compound was obtained in 13 % yield.
[0028] Weigh 0.4-0.6g nitrorosamine compound and dissolve it in 20-40mL methanol, add 0.6
Embodiment 2
[0033] Embodiment 2: solution preparation
[0034] Solution preparation: All reagents used in the experiment were of analytical grade and used directly without further treatment; the water used was secondary high-purity water, which was purified by a Milli-Q pure water machine.
[0035] Preparation of 1mM ligand standard solution: accurately weigh the compound Ros-N with an analytical balance with an accuracy of one ten-thousandth of a gram 3 Dilute the solid in a 10 mL volumetric flask with methanol to make a 1mM basic solution.
[0036] Preparation of 10mM anion standard solution: similar to the preparation of ligand standard solution, use an analytical balance to accurately weigh a certain amount of sodium salts of various anions in a 10 mL volumetric flask, dilute to volume with high-purity water, and prepare a concentration of 10 mmol / L The solution.
[0037] In the recognition system, the concentration of ligand is 10 μM, and the concentration of interfering ions and Na
Embodiment 3
[0038] Embodiment 3: spectral analysis
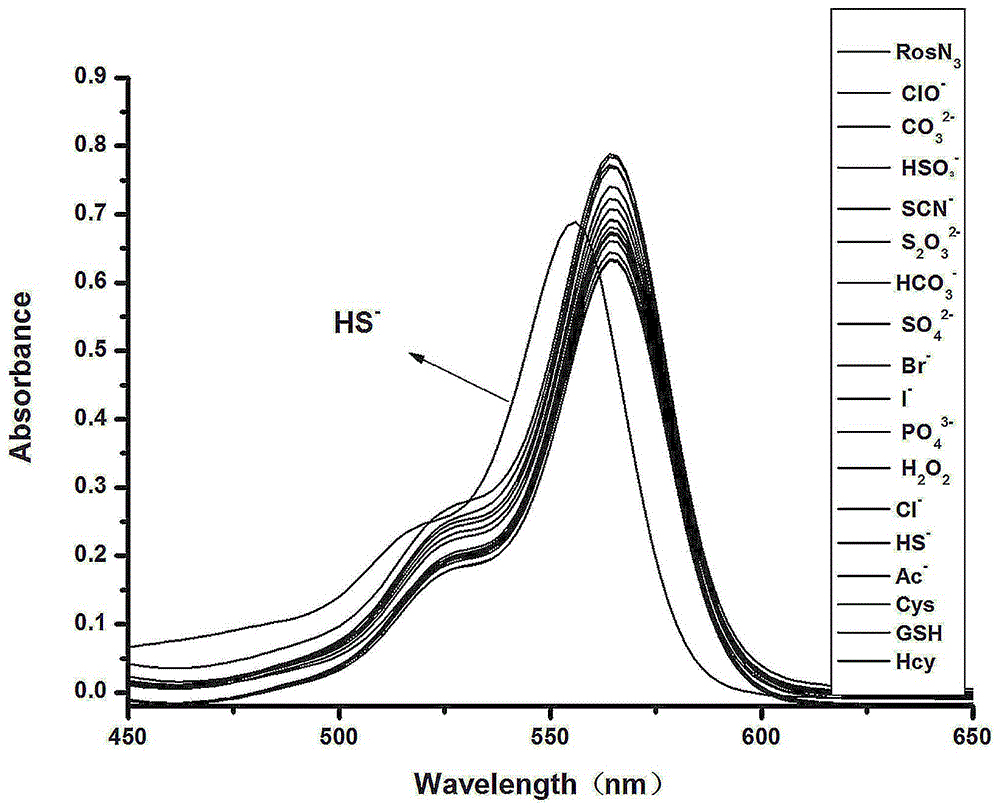
[0039] Separate Ligand Ros-N 3 There is a maximum absorption at 564nm, when adding 10 equivalents of HS - , there is a maximum absorption at 556nm. But when adding 10 equivalents of other ions (Br - , Cl - , F – ,ClO - , AcO - , CO 3 2- , HCO 3 - ,NO 3 - , SO 4 2- , HSO 3 - , SCN - ,S 2 o 3 2- , PO 4 3- ) and thiol derivatives (Hcy, Cys, GSH), there is no particularly obvious change in UV absorption. From the above results, it can be seen that ROSN 3 HS in DMF / PB ( 6 : 4 v / v, 10 mM, pH = 7.4 ) system - It has good UV absorption selectivity, see the results figure 1 .
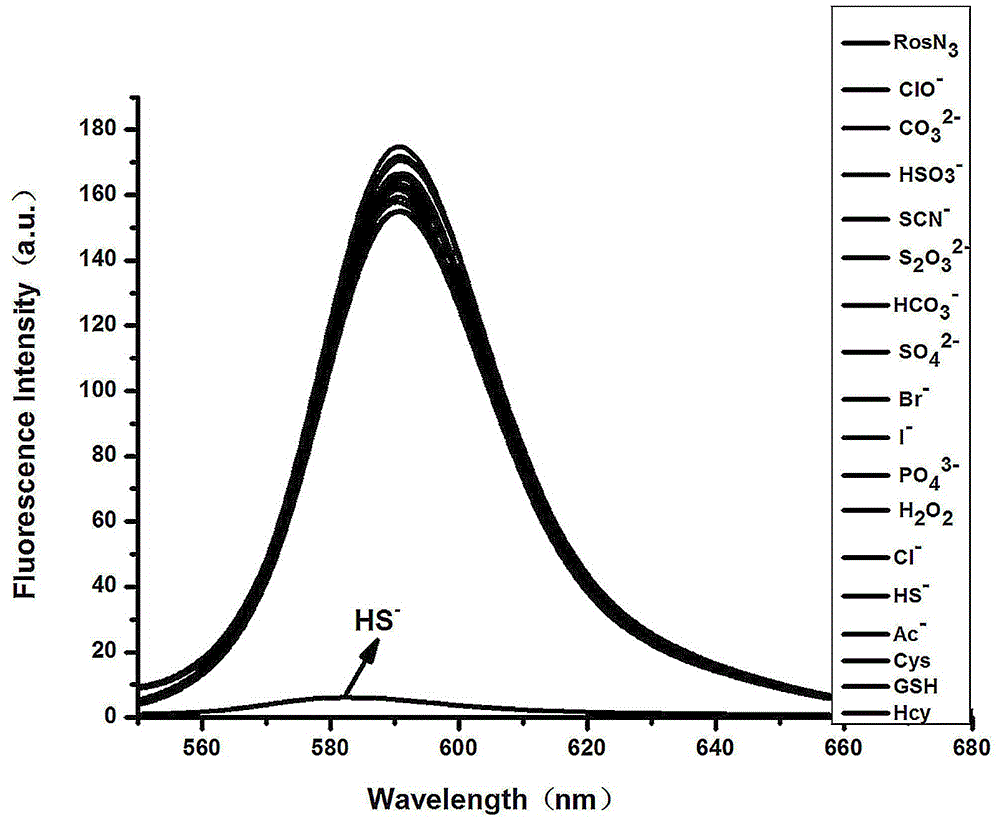
[0040] Probe Ros-N 3 Significant fluorescence at 589nm, when adding 10 equiv.HS - , the fluorescence is quenched. However, when other ions and small biomolecules are added, there is no particularly obvious change in fluorescence. From the above experimental results, it can be known that ROSN 3 HS in DMF / PB (6 : 4 v / v, 10 mM, pH = 7.4 )
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap