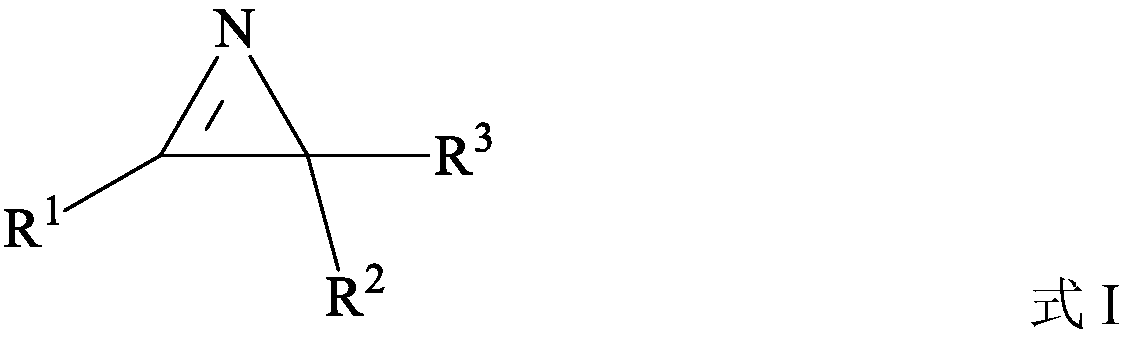Preparation method of nitrogen-containing heterocyclic compound
A technology for nitrogen heterocyclic compounds and azide compounds is applied in the field of chemistry, which can solve the problems of high cost and achieve the effects of non-biological toxicity of catalysts, wide and cheap raw material sources, and cheap catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Example 1 product 1-1
[0111] Under nitrogen protection, 4-propylphenylacetylene (0.5mmol), perfluoroiodobutane (0.75mmol), TMSN 3 (1.0mmol), oxide TBPB (1.0mmol), solvent (2mL) and catalyst Fe(OTf) 3 (12.7mg, 0.025mmol) was placed in a dry reaction tube and reacted at 20°C for 20 minutes. After the reaction was completed, the solvent was removed by a rotary evaporator under reduced pressure, dissolved in toluene, continued to react at 120° C. for 10 minutes, and separated by column chromatography to obtain the product 1-1.
Embodiment 2
[0112] Embodiment 2 product 1-2~1-32
[0113] The preparation method of the product in this example is the same as that in Example 1, see Table 1 for the differences.
[0114] Table 1
[0115]
[0116]
[0117]
[0118] Products 1-27:
[0119] The preparation method is the same as the preparation method of product 1-1, the difference is:
[0120] After being dissolved in toluene, the reaction temperature was 110° C., and the reaction time was 15 minutes.
[0121] Products 1-28:
[0122] The preparation method is the same as the preparation method of product 1-1, the difference is:
[0123] After being dissolved in toluene, the reaction temperature is 130° C., and the reaction time is 5 minutes.
[0124] Products 1-29:
[0125] The preparation method is the same as the preparation method of product 1-1, the difference is:
[0126] Before dissolving by methanol, the reaction temperature in the reaction tube was -20°C, and the reaction time was 30 minutes.
[0127]
Embodiment 3
[0137] Example 3 Structural Characterization
[0138] In this example, the structures and yields of products 1-1 and 1-32 were analyzed, and the specific structures are shown in Table 2.
[0139] Table 2
[0140]
[0141]
[0142]
[0143]
[0144]
[0145]
[0146] Products 1-27 to 1-32 were subjected to the same test as the above-mentioned products, and the results were similar to those of product 1-1 in Table 1: the target products were all obtained.
[0147] The shape of the products 1-1 to 1-26 is: clear oil.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap