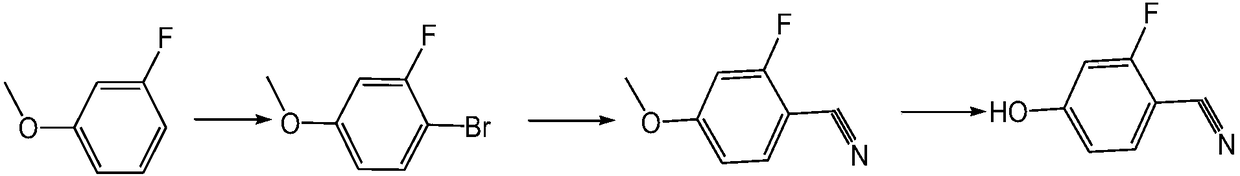
Preparation method of 3-fluoro-4-cyanophenol
A technology of cyanophenol and difluorobenzonitrile, which is applied in the field of organic chemical synthesis, can solve problems such as difficulty in realizing industrialized production, high environmental pollution, and poor product purity, and achieve a reduction in the proportion of by-products, good purity, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
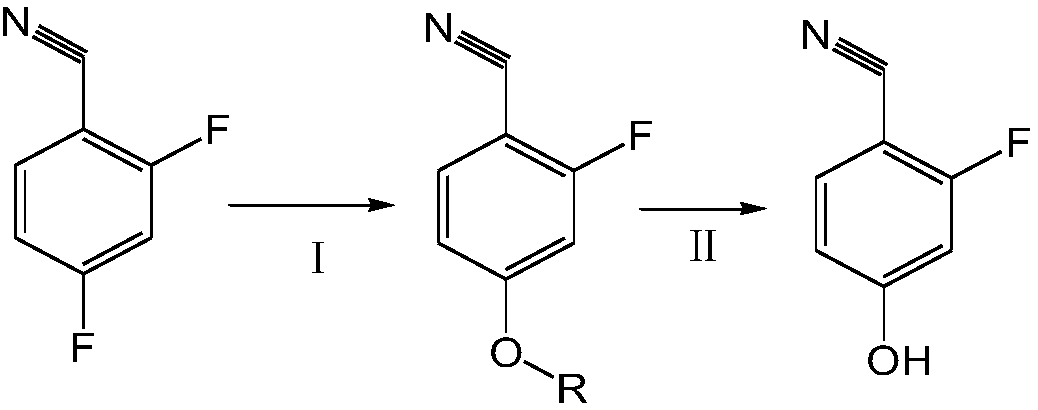
[0024] A preparation method of 3-fluoro-4-cyanophenol, which adopts 2,4-difluorobenzonitrile to obtain 2-fluoro-4-alkoxybenzonitrile through substitution reaction, and then obtains 3-fluorobenzonitrile through hydrolysis reaction -4-cyanophenol, its synthetic route is as follows:
[0025]
[0026] (I) Substitution reaction
[0027] Add 2,4-difluorobenzonitrile, catalyst and solvent into the reaction vessel and stir. The solvent is any one of tetrahydrofuran, dioxane, dimethyl sulfoxide or methyl tetrahydrofuran. The amount of solvent added 2 to 4 times the mass of the reactant; the catalyst is β-cyclodextrin or a mixture of β-cyclodextrin and starch with a mass ratio of 1 to 5:1, and the amount of catalyst added is 1 to 3% of the mass of the reactant, and then Add sodium alkylate in 2 to 4 times, the sodium alkylate is any one of sodium tert-butoxide, sodium isopropoxide, sodium tert-amyloxide or sodium n-butyloxide, 2,4-difluorobenzene The molar ratio of forminonitrile and
Embodiment 1
[0031] (I) Substitution reaction: Add the reactant 2,4-difluorobenzonitrile, catalyst and tetrahydrofuran into the reaction vessel, the amount of tetrahydrofuran added is twice the mass of the reactant, and the catalyst is β-cyclodextrin and starch according to the mass ratio 3:1 mixture, the amount of catalyst added is 2% of the mass of reactant, heat preservation at 10°C, add sodium tert-butoxide in 2 times, the total amount of sodium tert-butoxide added is the molar weight of 2,4-difluorobenzonitrile 1.1 times of 2-fluoro-4-alkoxybenzonitrile was obtained after 3 hours of reaction. The yield of this step is 95%, and the product purity is 85%.
[0032] (II) Hydrolysis reaction: Add 35% hydrochloric acid to the reaction solution containing 2-fluoro-4-alkoxybenzonitrile, the molar ratio of hydrochloric acid to 2-fluoro-4-alkoxybenzonitrile is 1 :8, slowly drop the toluene solution of 2-fluoro-4-alkoxybenzonitrile at 30°C, the molar ratio of 2-fluoro-4-alkylbenzonitrile to toluen
Embodiment 2~5
[0034] Embodiments 2-5 adopt the same method steps as the above-mentioned embodiment 1, and the different process parameters are shown in Table 1. The implication represented by the abbreviations that appear in the table is: "catalyst (m%)" represents the mass fraction that catalyst accounts for reactant in step (I), and " reactant: sodium alkyl alkoxide (mol) " refers to 2,4-di The mol ratio of fluorobenzonitrile and sodium alkyl alkoxide; "Raw material: inorganic acid (mol)" in the step (II) refers to the mol ratio of 2-fluoro-4-alkoxybenzonitrile and inorganic acid. "Insulation time" refers to the incubation reaction time after adding raw materials.
[0035] Table 1
[0036]
[0037]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap