Method for converting N-acetylneuraminic acid hydrate into N-acetylneuraminic acid
A technology of acetylneuraminic acid and hydrate, which is applied in the field of chemical engineering, can solve problems such as unsuitable application fields, achieve low cost, ensure purity, and meet the effects of market demand
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0016] Example 1:
[0017] The method for converting N-acetylneuraminic acid hydrate into N-acetylneuraminic acid in this embodiment is as follows:
[0018] 1. Select N-acetylneuraminic acid dihydrate as raw material, and the purity of N-acetylneuraminic acid dihydrate is 98.1%;
[0019] 2. Weigh 10g of the above-mentioned N-acetylneuraminic acid dihydrate and add a certain amount of purified water to prepare an aqueous solution of N-acetylneuraminic acid dihydrate with a concentration of 30g / L;
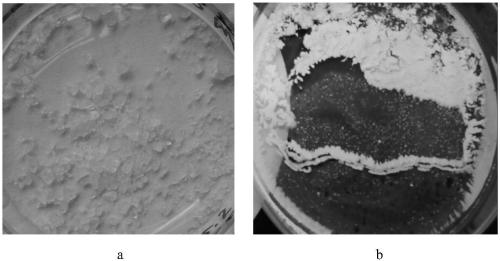
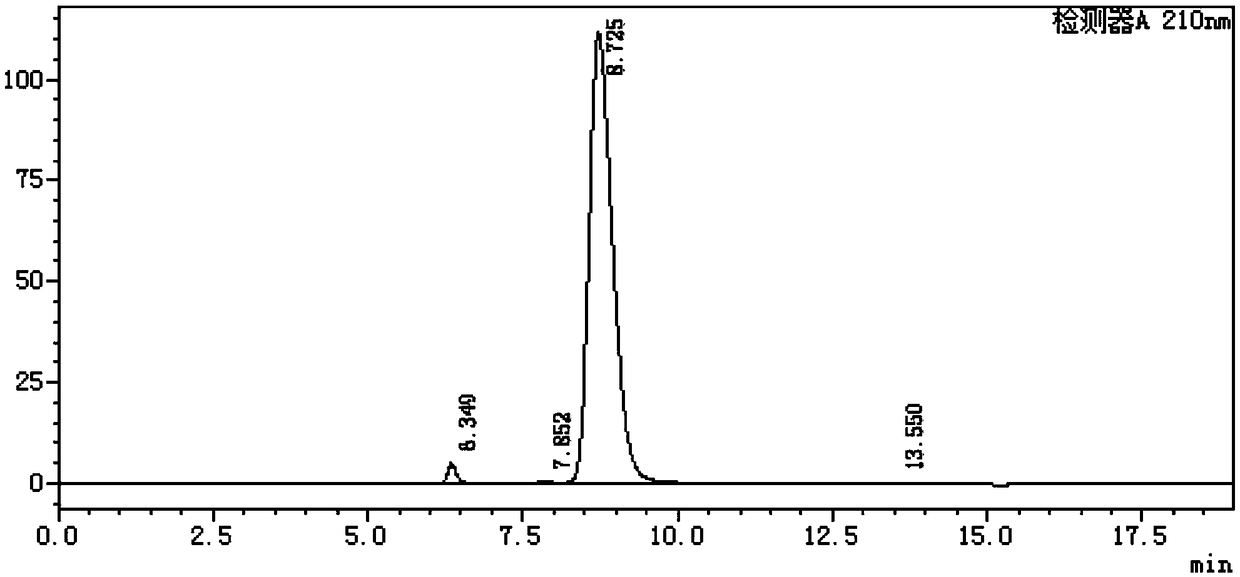
[0020] 3. Put the N-acetylneuraminic acid dihydrate aqueous solution prepared in step 2 into a drying box, vacuum to -0.1MPa, and heat to 50°C to evaporate the water in the aqueous solution, and finally dry the material. The dried material was tested to be N-acetylneuraminic acid and the content was 98.0%. Crystals after drying (appearance like figure 1 Shown) Detected by high performance liquid chromatography (results are as figure 2 Shown).
Example Embodiment
[0021] Example 2:
[0022] In this embodiment, the method for converting N-acetylneuraminic acid hydrate into N-acetylneuraminic acid is as follows:
[0023] 1. Select N-acetylneuraminic acid dihydrate as raw material, and the purity of N-acetylneuraminic acid dihydrate is 98.5%;
[0024] 2. Weigh 10g of the above-mentioned N-acetylneuraminic acid dihydrate and add a certain amount of purified water to prepare an aqueous solution of N-acetylneuraminic acid dihydrate with a concentration of 40g / L;
[0025] 3. Put the N-acetylneuraminic acid dihydrate aqueous solution prepared in step 2 into a drying box, vacuum to -0.1MPa, and heat to 60°C to evaporate the water in the aqueous solution, and finally dry the material. The dried material was tested to be N-acetylneuraminic acid and the content was 98.5%.
Example Embodiment
[0026] Example 3:
[0027] The method for converting N-acetylneuraminic acid hydrate into N-acetylneuraminic acid in this embodiment is as follows:
[0028] 1. Select N-acetylneuraminic acid dihydrate as the raw material, and the purity of N-acetylneuraminic acid dihydrate is 99.1%;
[0029] 2. Weigh 10g of the above-mentioned N-acetylneuraminic acid dihydrate and add a certain amount of purified water to prepare an aqueous solution of N-acetylneuraminic acid dihydrate with a concentration of 50g / L;
[0030] 3. Put the N-acetylneuraminic acid dihydrate aqueous solution prepared in step 2 into a drying box, vacuum to -0.1MPa, and heat to 70°C to evaporate the water in the aqueous solution, and finally dry the material. The dried material was tested to be N-acetylneuraminic acid and the content was 99.0%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap