Application of pseudostrychnine to inhibiting human colon cancer cells
A technology of colon cancer cells and strychnine, which is applied in the field of chemical medicine, can solve the problems of unreported application of pseudo strychnine, and achieve the effect of inhibiting proliferation and promoting apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
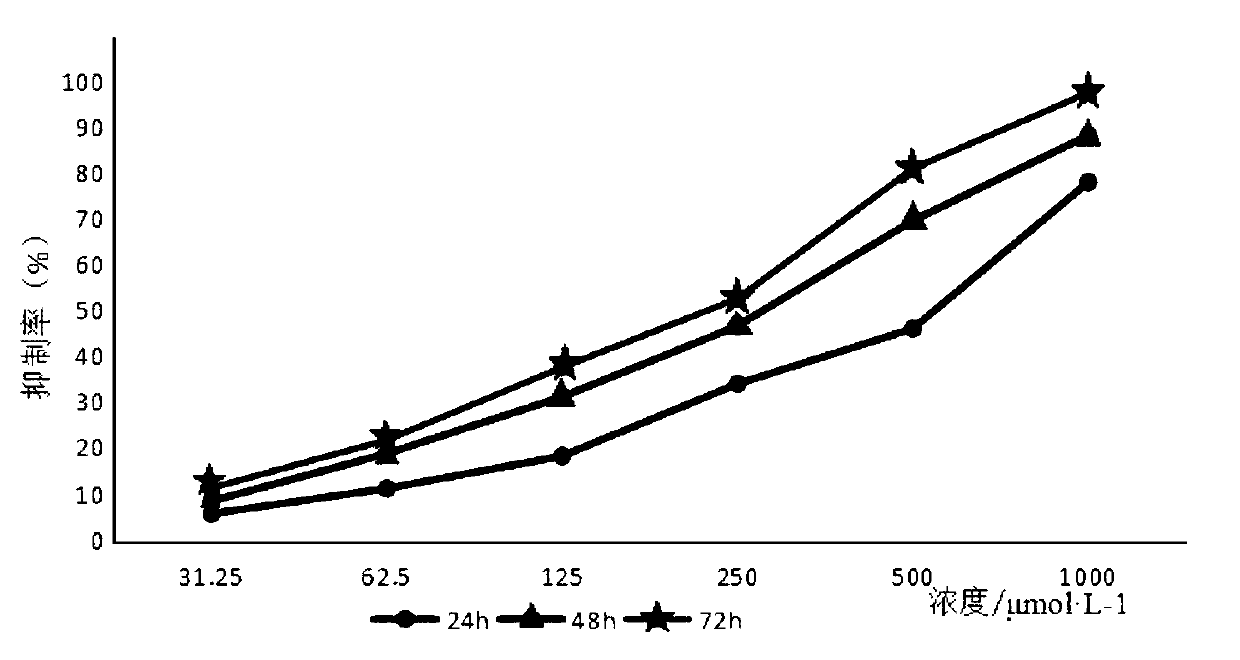
[0018] Example 1: Anti-proliferation experiment of pseudostychnine on HT-29 cells
[0019] 1. Cell culture
[0020] Take the HT-29 cells out of the liquid nitrogen, quickly put them into a 37°C water bath, shake the cryopreservation tube gently to dissolve the cryopreservation solution, transfer the cells to a centrifuge tube containing 5mL medium after dissolution, and collect the cells by centrifugation. Room temperature 1000r·min -1 Discard the supernatant after centrifugation for 5 minutes; suspend the cells with MC5A complete medium containing 10% fetal bovine serum, inoculate them into a petri dish, and gently pipette to mix well, at 37°C, 5% CO 2 cultured under saturated humidity conditions. Cells were passaged when the cell density reached 80%.
[0021] 2. MTT test
[0022] Take HT-29 cells in logarithmic growth phase and in good growth state, adjust the cell density to 3×10 with MC5A complete medium containing 10% fetal bovine serum 4 cells / mL, after inserting int
Embodiment 2
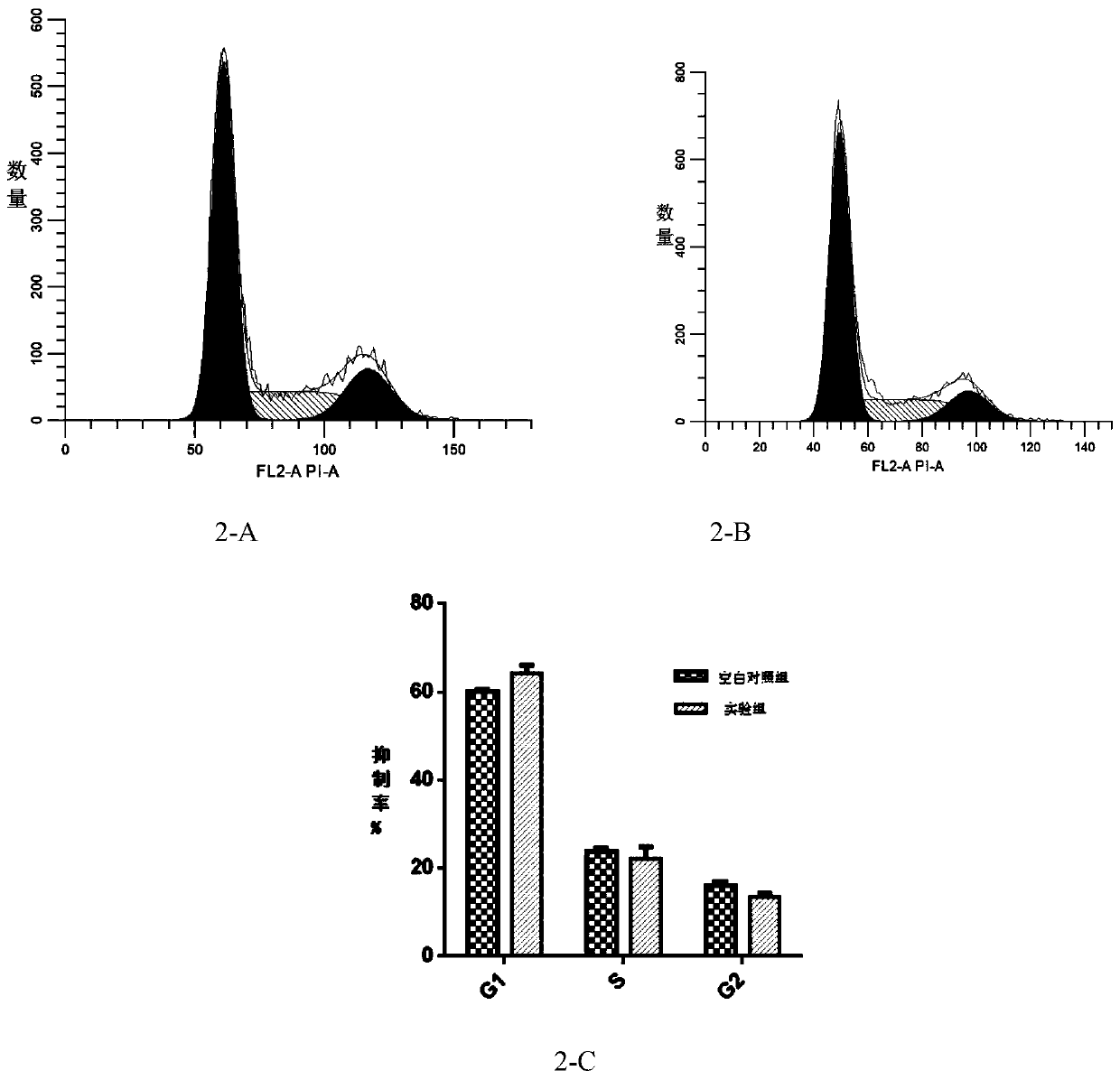
[0031] Embodiment 2: Effect experiment of pseudostychnine on the cell cycle of HT-29 cells
[0032] Take the HT-29 cells that are in the logarithmic growth phase and in a good growth state cultivated in Example 1, and use 2.5×10 5 Cells were seeded in a 6-well plate for cell culture at 37°C, 5% CO 2 Incubate overnight in the incubator. Set the pseudo-stychnine concentration to 0 μmol L -1 The concentration of blank control group and pseudo strychnine was 250 μmol L -1 The experimental group, 37 ℃, 5% CO 2 After culturing in the incubator for 48 hours, the cells were collected. Digest cells with 0.25% trypsin without EDTA, collect cells after termination of digestion, 1000r·min -1 Centrifuge for 5 minutes, remove the supernatant, resuspend and wash with PBS buffer twice, and then centrifuge again to remove the supernatant. Take 100 μL of PBS to resuspend the cells, slowly add 700 μL of pre-cooled 80% ethanol to make the final concentration of ethanol to 70%, and fix at 4°C f
Embodiment 3
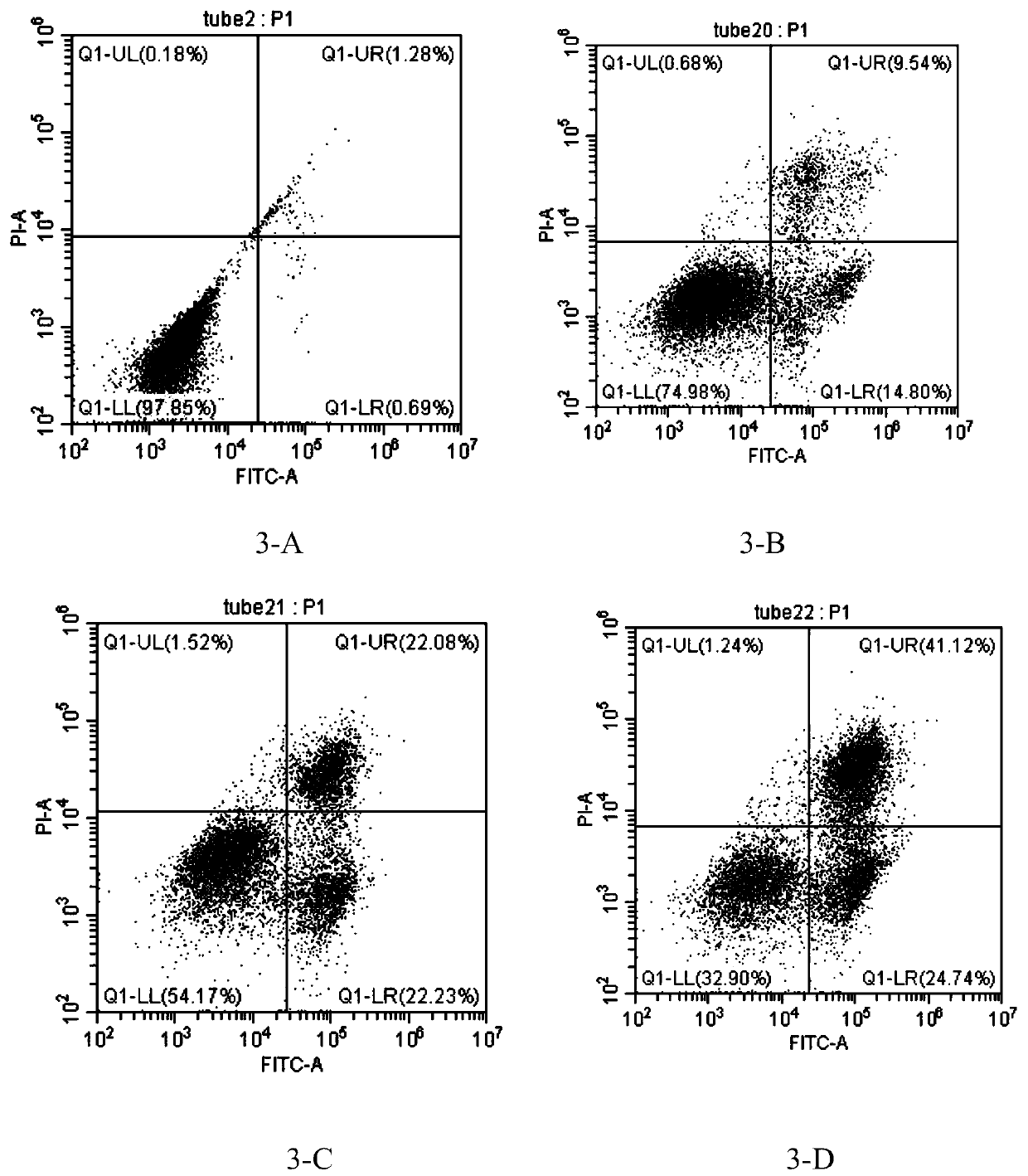
[0034] Example 3: Effect of different concentrations of pseudostychnine on the apoptosis of HT-29 cells
[0035] 1. Cell culture
[0036] HT-29 cells were cultured in complete medium containing 10% fetal bovine serum, MC5A+10% FBS+1% penicillin-streptomycin mixed solution, placed at 37°C, 5% CO 2 Incubate and cultivate under saturated humidity conditions in a constant temperature incubator.
[0037] 2. Grouping and administration
[0038] A blank control group without pseudostychnine and three experimental groups with different concentrations of pseudostychnine were set up. The HT-29 cells in logarithmic phase growth were randomly divided into 4 parts, 3 of which were added with pseudo-stychnine at concentrations of 125 μmol / L, 250 μmol / L, and 500 μmol / L respectively, as the experimental group, and the other group was added with the experimental group. The same dose of culture medium in the group was used as the blank control group, and each group had three replicate wel
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap