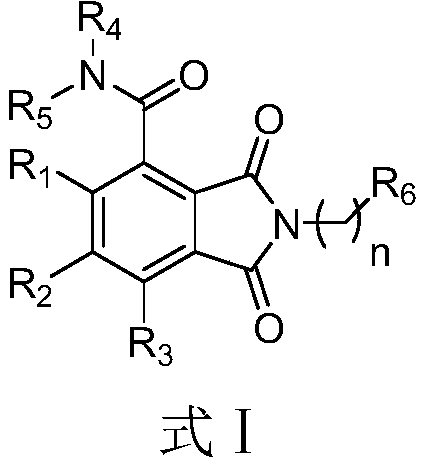
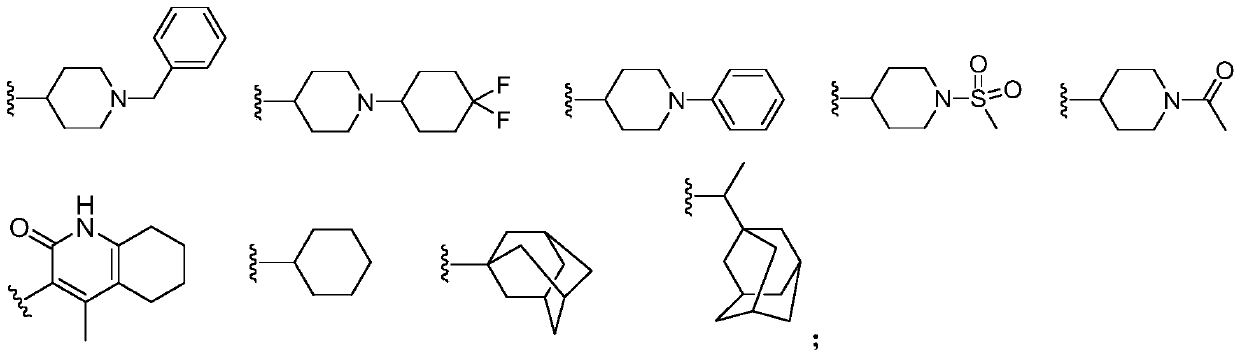
Phthalimide derivative as well as preparation method and application thereof
A phenyl and methyl technology, which can be used in drug combinations, pharmaceutical formulations, medical preparations containing active ingredients, etc., and can solve problems such as affecting anti-tumor effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
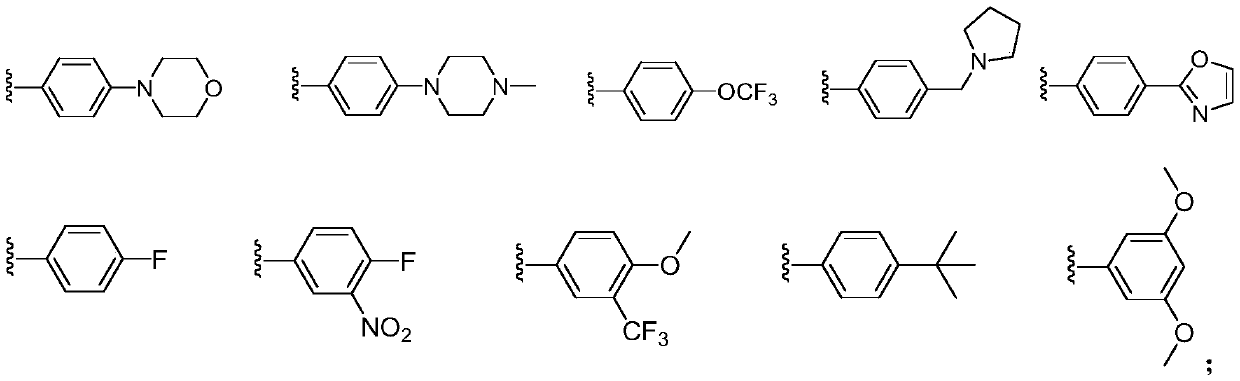
[0103] Example 1 Compound H1: 2-(4-morpholinylphenyl)-1,3-dioxoisoindole-4-carboxamide
[0104] The first step, the preparation of 3-carboxyphthalic anhydride (2)
[0105]
[0106] 2.1 g (10 mmol) of 1,2,3-benzenetricarboxylic acid (1) and 30 mL of THF were added to a 100 mL single-necked round-bottomed flask, shaken and shaken. The solvent was distilled off under reduced pressure to make the raw material adherent. Put the open-top round-bottomed flask into a 150°C oil bath for dry burning for 6 hours, and during the process, it is obvious that water droplets are formed on the bottle wall. After the completion of the reaction, the reaction was lowered to room temperature to obtain 21.9 g of white solid with a yield of 99%; 1 H NMR (400MHz, DMSO-d 6 )δ: 7.77(d, J=7.7Hz, 2H), 7.44(t, J=7.7Hz, 1H); 13 C NMR (101MHz, DMSO-d 6 )δ: 169.92, 169.07, 136.26, 131.57, 129.04.
[0107] The second step, the preparation of 2-(4-morpholinylphenyl)-1,3-dioxoisoindole-4-carboxylic acid (
Example Embodiment
[0113] Example 2 Compound H2: 2-(4-(4-methylpiperazin-1-yl)phenyl)-1,3-dioxoisoindole-4-carboxamide
[0114]
[0115] The reaction conditions for preparing compound H2 are similar to those of compound H1, and the second step of the reaction uses 3-carboxyphthalic anhydride and 4-(4-methylpiperazine)aniline as raw materials. Solid (39.2% yield). 1 H NMR (400MHz, Chloroform-d) δ: 9.98 (s, 1H), 8.76 (dd, J=8.0, 1.2Hz, 1H), 8.13 (dd, J=7.3, 1.2Hz, 1H), 7.92 (t, J=7.7Hz, 1H), 7.27(d, J=6.9Hz, 2H), 7.07~7.00(d, J=8.9Hz, 2H), 6.00(s, 1H), 3.29(t, J=5.1Hz, 4H), 2.61~2.56(m, 4H), 2.37(s, 3H); 13 C NMR (101MHz, DMSO-d 6 )δ: 168.11, 167.08, 166.88, 138.55, 135.21, 134.57, 134.41, 134.21, 133.68, 130.24, 129.51, 125.42, 120.77, 57.54, 53.92, 44.32; HR-MS (ESI-Calc for C for C) m / z 20 H 20 N 4 O 3 {[M+H] +}365.1608, found365.1610.
Example Embodiment
[0116] Example 3 Compound H3: 2-(4-(trifluoromethoxy)phenyl)-1,3-dioxoisoindole-4-carboxamide
[0117]
[0118] The reaction conditions for preparing compound H3 are similar to those of compound H1, and the second step reaction uses 3-carboxyphthalic anhydride and 4-trifluoromethoxyaniline as raw materials. Solid (47.3% yield). 1 H NMR (400MHz, DMSO-d 6 )δ13.40(s, 1H), 8.39(s, 1H), 8.35–8.23(m, 2H), 7.91(dd, J=8.6, 1.4Hz, 1H), 7.48(s, 2H), 7.25(d , J=5.1Hz, 1H), 7.14(t, J=7.8Hz, 1H), 6.94(d, J=7.5Hz, 1H), 6.88(t, J=1.9Hz, 1H), 6.74(s, 2H ),6.53(dd,J=8.0,2.2Hz,1H),5.66(d,J=5.1Hz,1H),2.76(d,J=4.9Hz,3H). 13 C NMR (101MHz, DMSO-d 6 )δ164.34,164.15,159.51,150.68,143.03,142.06,138.03,135.62,131.25,129.60,122.25,121.43,119.98,119.92,114.78,112.44,109.64,109.27,106.78,30.30.HRMS(ESI-TOF)m / z Calcd for C 20 H 19 N 6 [M+H] + :343.1672,found:343.1678.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap