Recombinant protein and fragments thereof, method for producing said recombinant protein, synthetic gene and use of the recombinant protein
A technology of recombinant protein and synthetic gene, which is applied in the fields of pharmacy, molecular biology, medicinal chemistry, biochemistry and genetics, and can solve problems such as short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
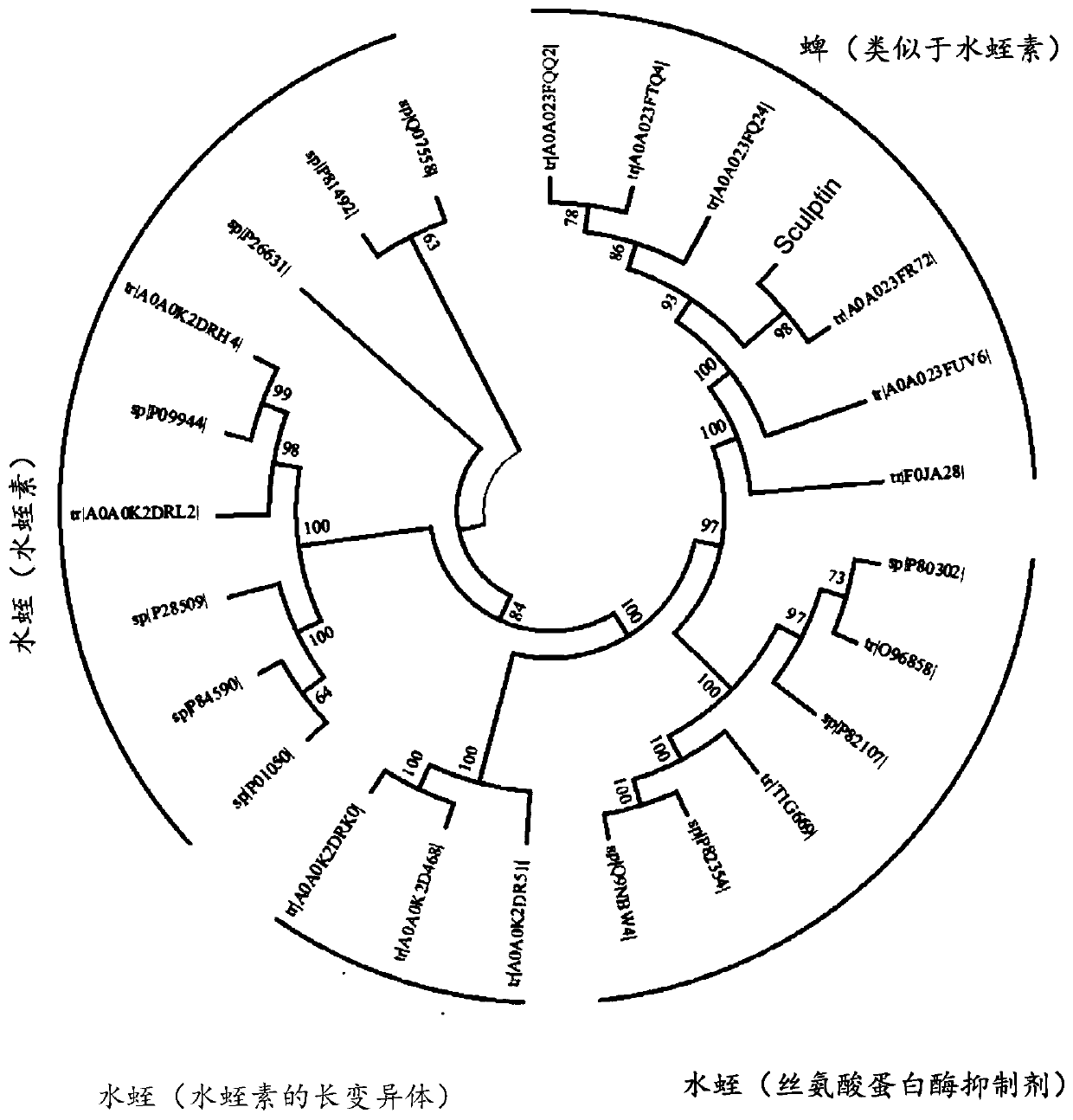
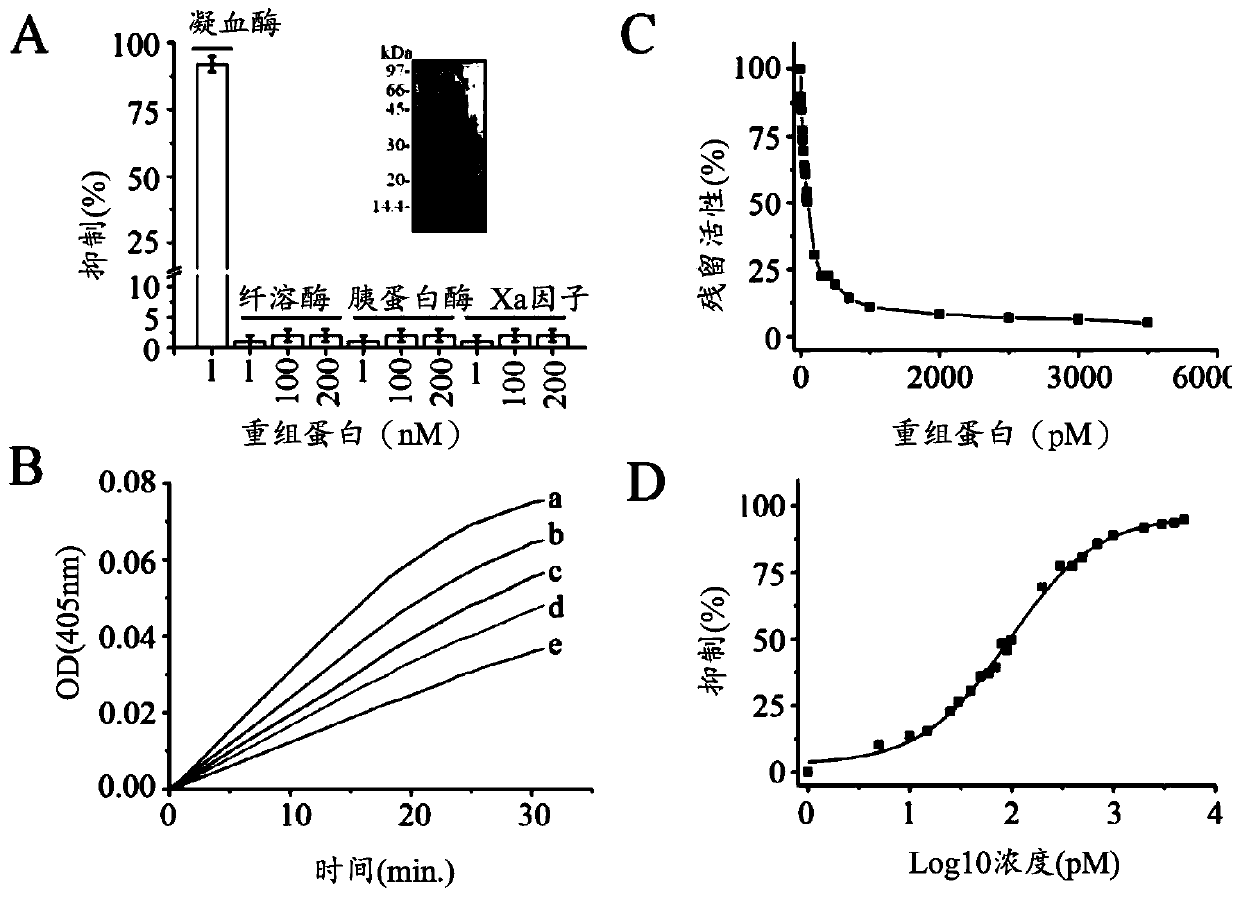
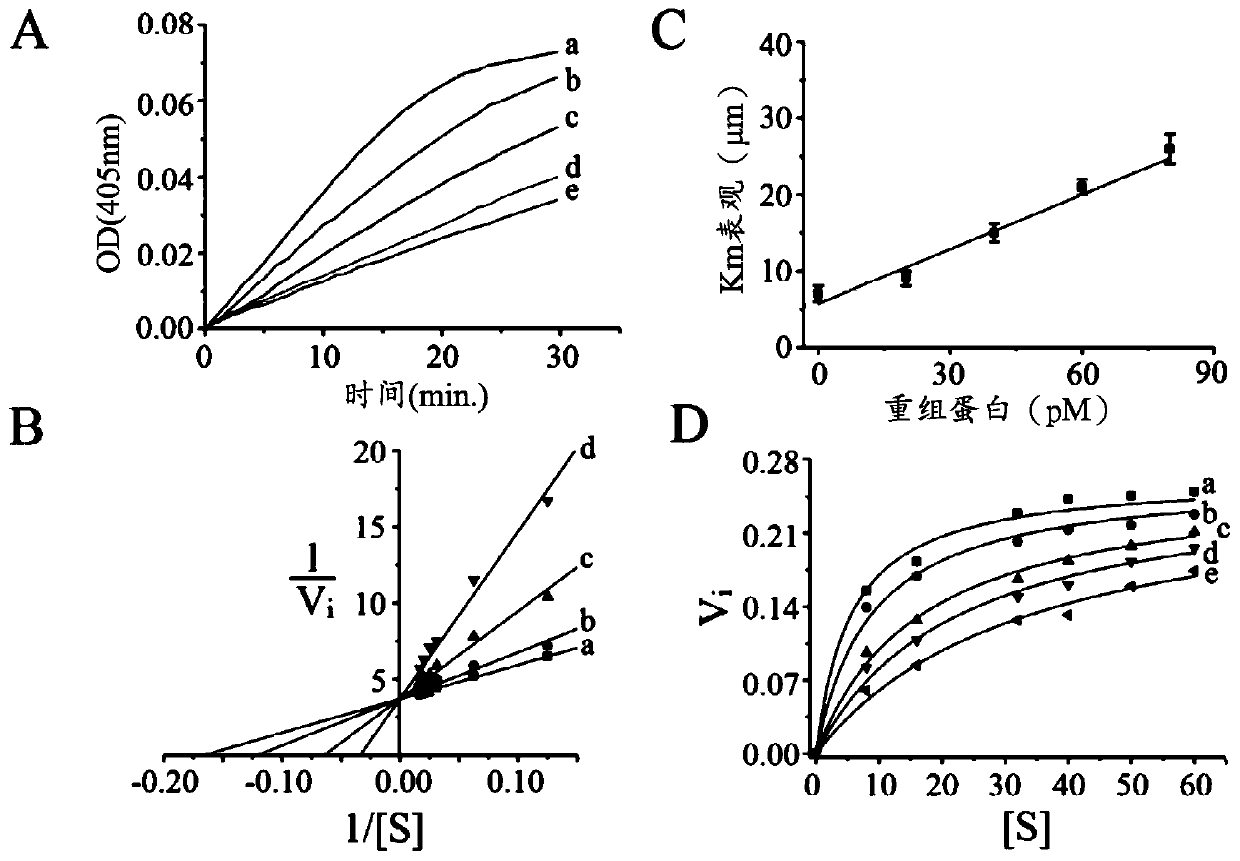
[0073] Here, a novel class of thrombin inhibitors, in particular direct and specific thrombin inhibitors modified from sculptin identified in transcriptome analysis of tick salivary glands, will be described. It consists of 168 residues with four identical repeats and exhibits evolutionary divergence from classical hirudins. The recombinant protein is a competitive, specific and reversible thrombin inhibitor with a K of 18.5±2.2pM. It is slowly digested by thrombin and loses its inhibitory activity. Thus, the recombinant protein is hydrolyzed by Factor Xa, and each polypeptide fragment is capable of inhibiting thrombin in an independent manner. A single domain of the recombinant protein, which retains only about 45% of its inhibitory activity, has been proposed to bind thrombin in a bivalent fashion. Forming a helix / small turn-like structure by incorporating residues from the active site of the recombinant protein domain would make it the most potent thrombin inhibitor compar
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap