Method for determining content of impurities contained in etamsylate medicine and content of main medicine thereof
A technology for ethanamine and impurities, which is applied in the field of determination of the impurities contained in the drug and the content of the main drug, can solve the problem of low penetration rate, good repeatability and durability, and the polarity of ethanamine. Large and other problems, to achieve the effect of universal detection conditions, high detection sensitivity, and good accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0054] Example 1
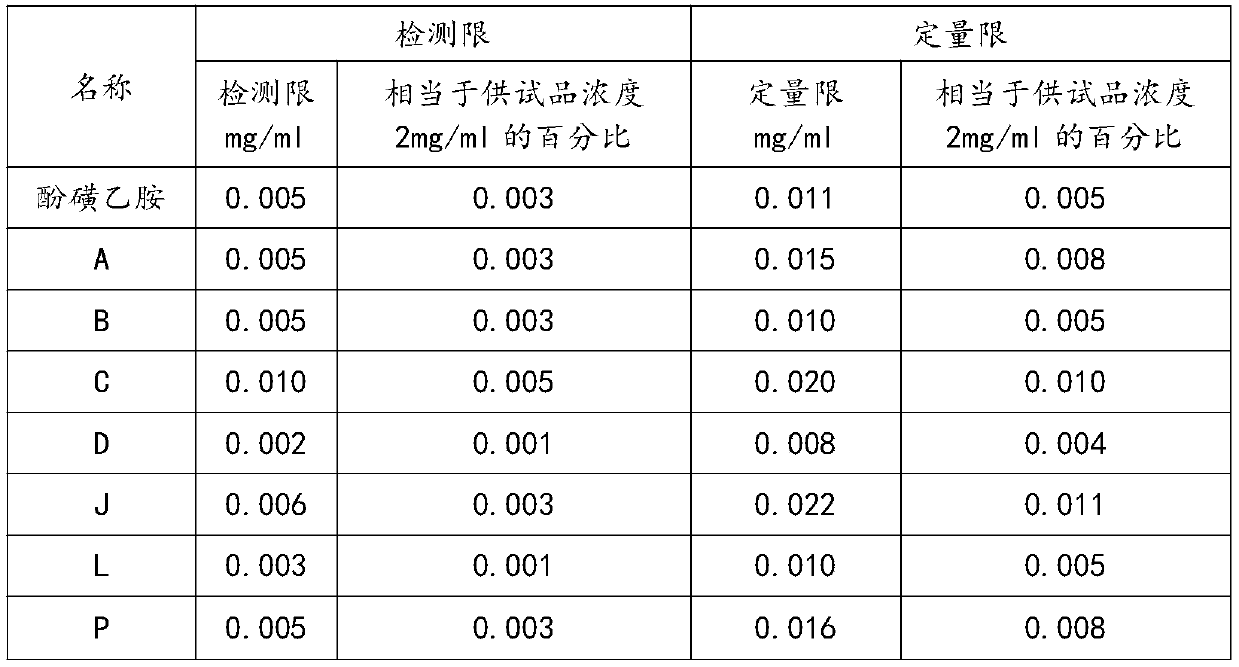
[0055] Limits of detection and limits of quantitation
[0056] Specifically include the following steps:
[0057] S11, configure each impurity mother liquor;
[0058] Accurately weigh 20 mg each of impurities A, B, C, D, and P, put them in 100ml measuring bottles, dissolve them with diluent, dilute to the mark and shake well;
[0059] Accurately weigh 20 mg each of impurities L and J, put them in a 100 ml measuring bottle, dissolve them with 1 ml of methanol first, then dissolve them with a diluent and dilute to the mark and shake well;
[0060] Accurately weigh 10 mg of ethylamine phensulfame, put it in a 10 ml measuring bottle, dissolve it with diluent and dilute to the mark and shake well.
[0061] S12, configuring each detection limit solution;
[0062] Accurately measure 0.25ml each of the above-mentioned impurity P, J, L, A, B mother liquor, 0.5ml of impurity C mother liquor, 0.1ml of impurity D mother liquor, and 1ml of ethylamine sulfamate mother li
Example Embodiment
[0070] Example 2
[0071] Detection of Standard Curve
[0072] Specifically include the following steps:
[0073] S21, configuring the mother liquor of each impurity reference substance;
[0074] Accurately weigh 20 mg each of impurities A, B, C, D, and P, put them in 100ml measuring bottles, dissolve them with diluent, dilute to the mark and shake well;
[0075] Precisely weigh 20 mg each of impurities L and J, put them in a 100ml measuring bottle, first dissolve them with 1ml methanol, then dissolve them with diluent and dilute to the mark and shake well;
[0076] Accurately weigh 10 mg of ethylamine phensulfate and put it in a 50 ml measuring bottle, dissolve it with a diluent and dilute to the mark and shake well.
[0077] S22, configure each linear solution;
[0078] Linear solution 1: Accurately measure 1.0ml of the mother solution of each impurity reference substance and put it in a 100ml measuring bottle, dissolve it with a diluent and dilute to the mark and shake wel
Example Embodiment
[0092] Example 3
[0093] Accuracy test
[0094] Specifically include the following steps:
[0095] S31, configuring each impurity mother liquor;
[0096] Accurately weigh 20.0mg each of impurities A, B, C, D, and P, put them in 100ml measuring bottles, dissolve them with diluent and dilute to the mark and shake well;
[0097] Accurately weigh 20.0 mg of impurities L and J, put them in 100 ml measuring bottles, dissolve them with 1 ml of methanol first, then dissolve them with diluent and dilute to the mark and shake well.
[0098] S32, configure the recovery rate impurity mixed solution;
[0099] 50% impurity mixed solution: accurately measure 0.25ml of the above-mentioned impurity mother liquors, put them in a 100ml measuring bottle, dilute to the mark with a diluent, and shake well to obtain a 50% impurity mixed solution;
[0100] 100% impurity mixed solution: accurately measure 0.5ml of the above-mentioned impurity mother liquors, put it in a 100ml measuring bottle, dilut
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap