Preparation method of lithium difluorophosphate
A technology of lithium difluorophosphate and ethyl acetate, which is applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve problems such as high water content, low purity, and unsuitable conditions for lithium difluorophosphate, and achieve the goal of moisture content The effect of low cost, high purity and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
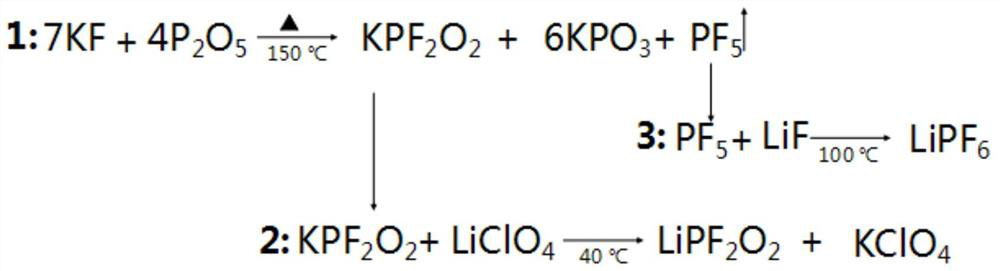
[0033] The invention provides a preparation method of lithium difluorophosphate, by making RPF 2 o 2 with LiClO 4 The mixed reaction obtains lithium difluorophosphate,
[0034] Wherein, R is K or Na;
[0035] The RPF 2 o 2 with LiClO 4 The molar ratio is 1: (1.8~5).
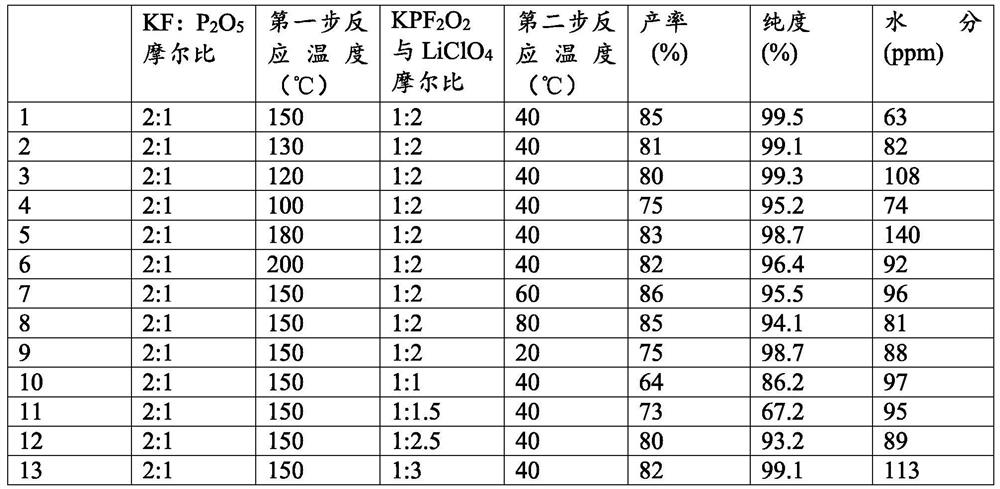
[0036] According to the present invention, the present invention will RPF 2 o 2 with LiClO 4 The mixed reaction obtains lithium difluorophosphate, wherein, the RPF 2 o 2 with LiClO 4 The molar ratio is preferably 1:(2~3), more preferably 1:(2.5~2.8); the temperature of the reaction is preferably 20~80°C, more preferably 30~70°C, most preferably 40~60°C ℃; the time of the reaction is preferably 6 to 10 hours, more preferably 8 to 9 hours; the present invention has no special requirements for the reaction device, and any device known in the art for the mixed reaction of inorganic substances can be used, as can It is a solid phase reactor; in the mixed reaction of the present invention, the reaction als...
Embodiment 1
[0044] 1) react in a solid-phase reactor, potassium fluoride and phosphorus pentoxide are KF according to the molar ratio of feeding: P 2 o 5 = 2: 1 feed, and adjust the reaction temperature in the kettle to 150 ° C, the reaction time 12h, to obtain RPF 2 o 2 and KPO 3 mixture of and PF 5 gas;
[0045] to RPF 2 o 2 and KPO 3 Ethylene glycol dimethyl ether (DME) is added to the mixture of the mixture, and the product is stirred and extracted, and enriched to obtain KPF-containing 2 o 2 DME solution
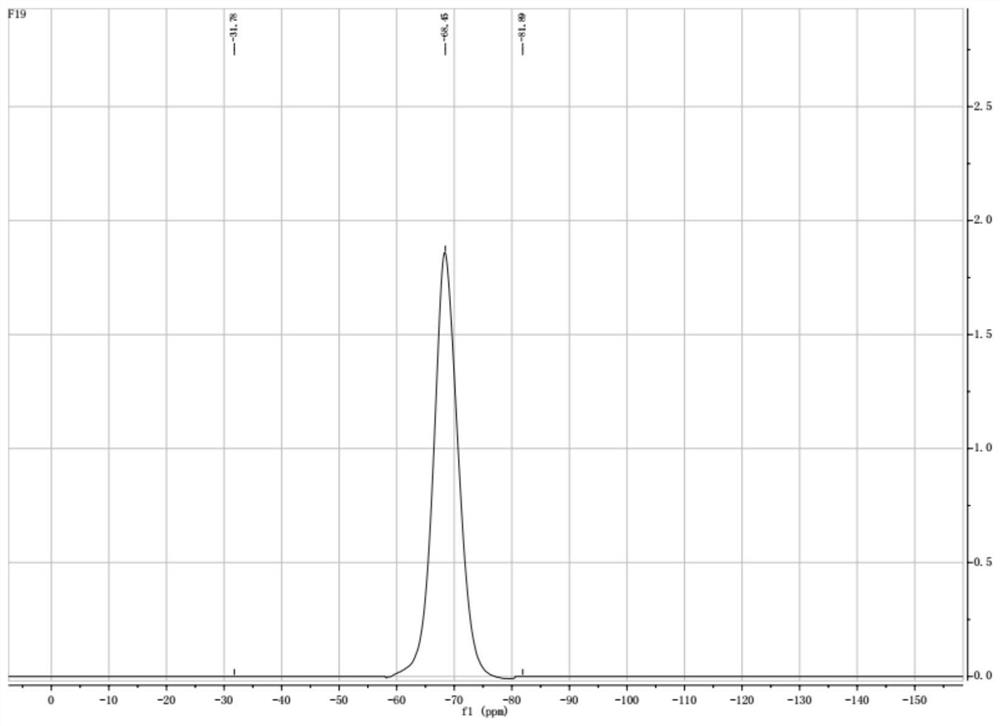
[0046] 2) According to KPF 2 o 2 with LiClO 4 The molar ratio is 1: 2 to carry out feeding, the reaction temperature is 40 ℃, the reaction time is 8h, the reaction solution obtained is in N 2 In the atmosphere, distill under reduced pressure at a temperature of 80°C and an air pressure of -0.1 to -0.2MPa for 6 hours, distill off DME and recover it, and dry the obtained crystals under reduced pressure at 80°C for 6 hours to obtain a white powder, which is Lithium diflu...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap