Preparation method and application of core-shell nanoparticles loaded with EBV-LMP2 mRNA
A technology of EBV-LMP2 and nanoparticles, which is applied in the field of core-shell nanoparticles and its preparation, can solve the problems that mRNA vaccines have not been reported in the literature, and achieve the effect of universality, small particle size and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 cationic liposome
[0041] Prepare LP by thin film dispersion-hydration-ultrasonic method, the specific method is as follows: DOTAP and Chol are prepared as a 10mg / mL stock solution with absolute ethanol, and DOTAP and Chol are added to a 1000mL spinner bottle at a ratio of 1:1 Add corresponding volume of absolute ethanol to 2mL. After the rotary evaporation, the spinner bottle was placed under vacuum conditions to further remove the organic solvent to prepare a lipid film. After film formation, add 2mL RNase-free water to hydrate the lipid film. After the hydration was completed, the obtained crude liposomes were transferred to a 10mL EP tube, and the crude LP solution was crushed with an ultrasonic cell disruptor, with the ultrasonic power set to 80W, ultrasonic for 3s, stop for 3s, and the total ultrasonic time was 3min. The LP obtained after sonication is obtained by filtering it with a 0.22 μm sterile water filter membrane. Further
Embodiment 2
[0042] Preparation and screening of embodiment 2 PLGA nanoparticles
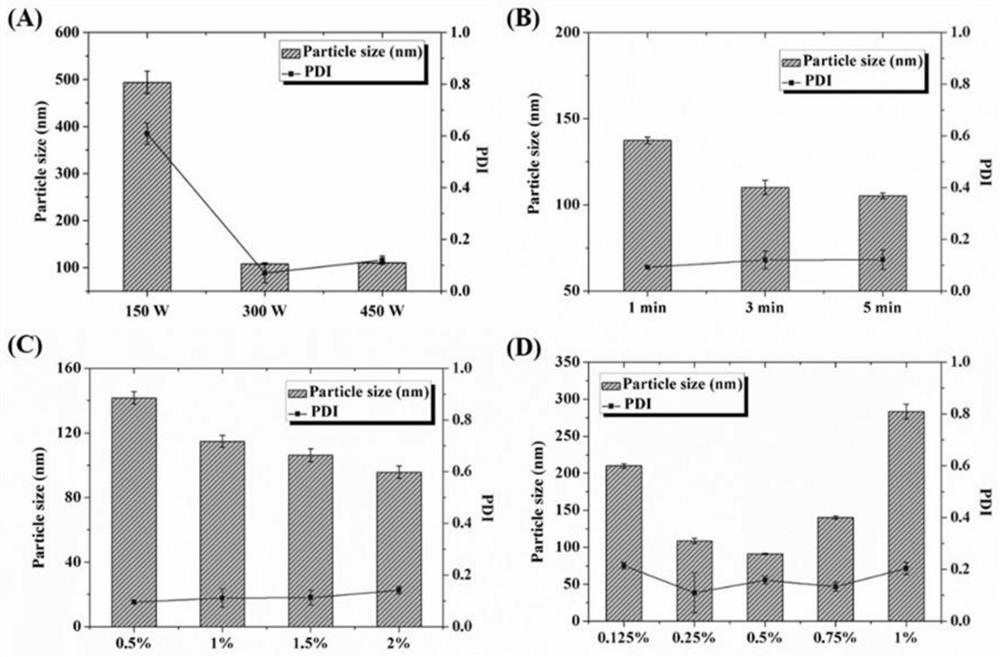
[0043] Weigh 20 mg of PLGA into a 2 mL EP tube, add 1 mL of ethyl acetate solvent to prepare the oil phase. At the same time, weigh 80 mg of PVA into a 10 mL EP tube, add 4 mL of 95° C. RNase-free water to prepare an aqueous phase solution containing 2% (w / v) of PVA. The oil phase was quickly injected into the water phase, and quickly placed in an ice bath for ultrasonic emulsification. The ultrasonic power was 300W, ultrasonic 3s, intermittent 3s, and the total ultrasonic time was set to 3min. After the ultrasonication, the emulsified solution was quickly transferred to an eggplant-shaped bottle, and was obtained by rotary evaporation at 75 rpm in a water bath at 37°C.
[0044] Ultrasound Power Screening
[0045] Weigh 20mg of PLGA and place it in a 2mL EP tube, add 1mL of ethyl acetate, and dissolve it ultrasonically in a water bath to prepare the oil phase. At the same time, weigh 40 mg of PVA into a 10
Embodiment 3
[0052] Preparation and screening of embodiment 3 core-shell nanoparticles
[0053] Ultrasound Power Screening
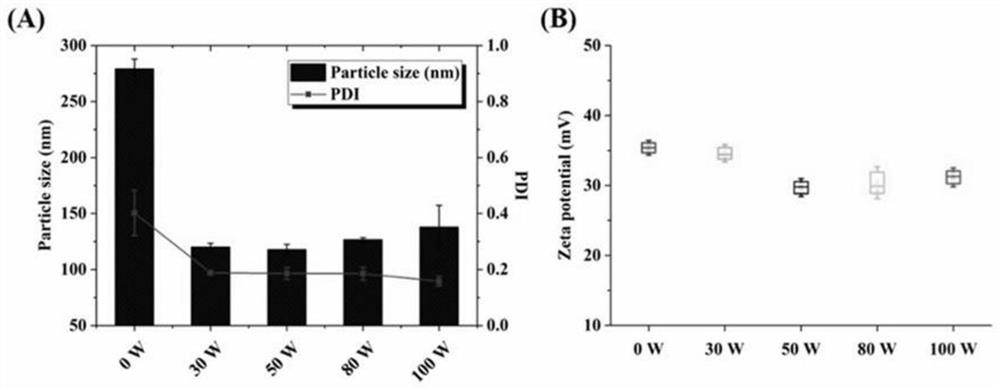
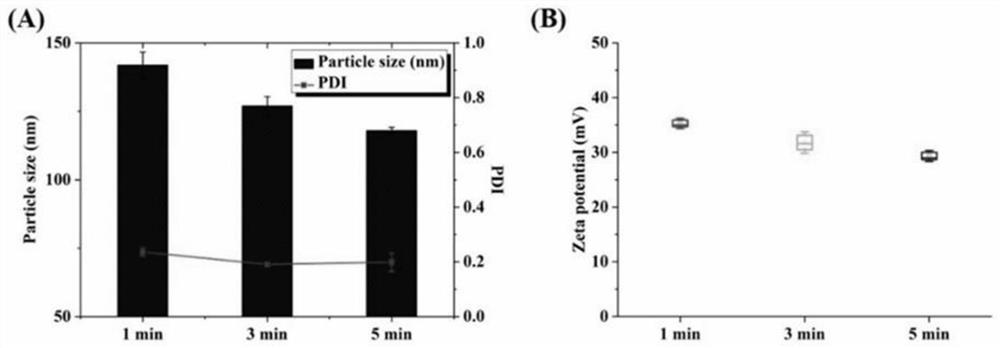
[0054] The lipid film was prepared according to the preparation method of Example 1, wherein the molar ratio of DOTAP to Chol was 1:1, and the total lipid concentration was 6 mM. Add 2 mL of PLGA nanoparticles prepared in Example 2 into the lipid film for hydration for 30 min, then transfer the hydrated solution to a 10 mL EP tube, and homogenize it by ultrasonic probe. The ultrasonic power was set to 30W, 50W, 80W and 100W respectively, the ultrasonic was 3s, the interval was 3s, and the total ultrasonic time was set to 3min. After the ultrasonic wave was finished, it was measured with a Malvern particle size potential analyzer, and the results are shown in the attached figure 2 .
[0055] It can be seen from the results that the diameter of the coarse core-shell nanoparticles after direct hydration is 279.2±8.8nm, and the PDI is 0.402±0.081. It can be seen that t
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap