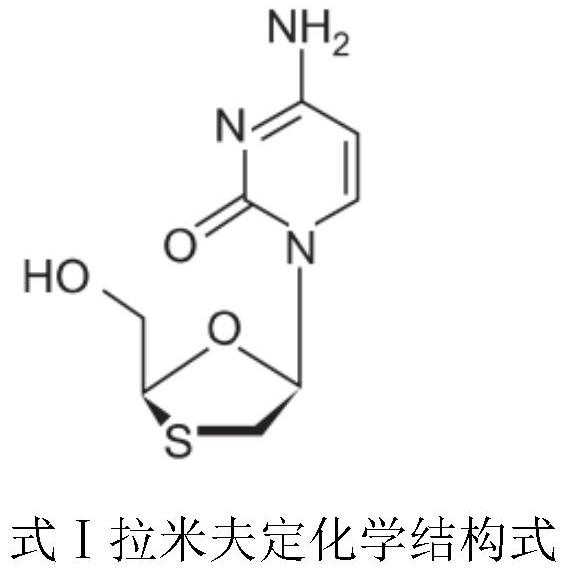
Method for determining contents of auxiliary materials in lamivudine tablet
A technology for lamivudine tablets and lamivudine tablets, which is applied in the field of determining the content of excipients in lamivudine tablets, and can solve performance differences such as tablet dissolution, content uniformity, and drug stability, and titration methods Problems such as manual operation and low efficiency of titration method can achieve the effect of reducing the probability of defective lamivudine tablets, good quality control of drugs, and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] A method for determining auxiliary auxiliary tablet in a lamifer tablet comprising the steps of:
[0074] S1 carboxymeta sodium content detection
[0075] S1.1 solution formulation
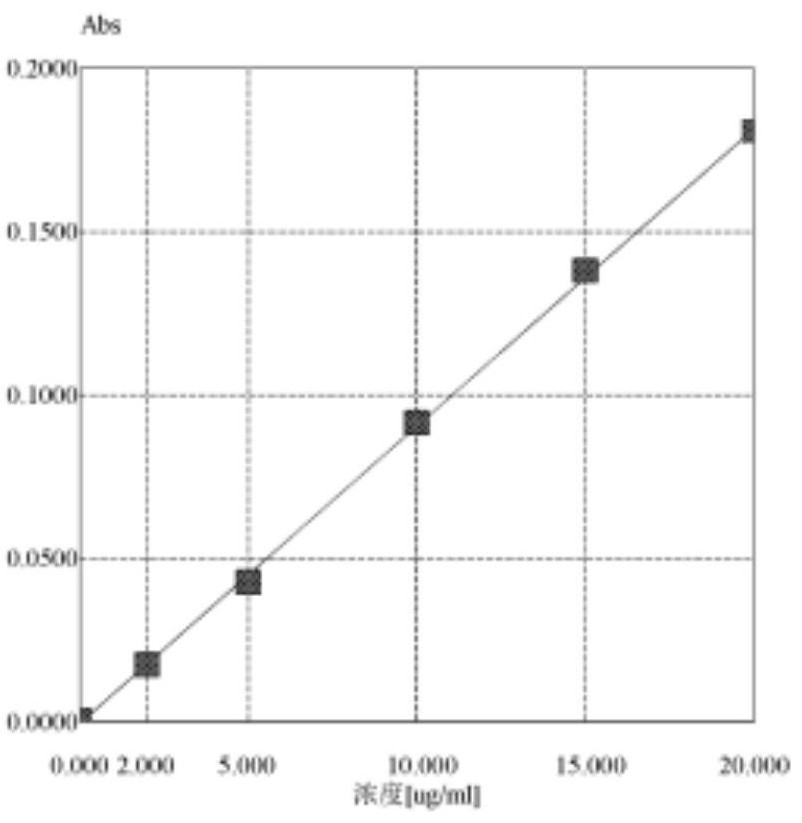
[0076] Standard curve solution : The precision amount of sodium standard solution is given, and the sodium containing sodium, 2 μg, 5 μg, 10 [mu] g, 2 μg, 5 μg, 10 μg, and 20 μg, the concentration is the abscissive, and the absorbance is longitudinally. Linear regression , Standard curve of sodium, as follows figure 1 As shown, it is a linear relationship of sodium.
[0077] by figure 1 It can be seen that sodium is in the concentration range of 0-20 μg / ml, with a concentration of a horizontal coordinate, the resulting regression curve is [A] = 0.0091 * [C] -0.0005; the correlation coefficient R is 0.99976, indicating that the analysis method has a good linear relationship in the range of 0-20 μg / ml concentration.
[0078] Test solution solution
[0079] Take 20 pieces of this product to
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap