Naphtho-silole compound and synthesis method for preparing naphtho-silole compound through photocatalysis
A synthesis method and compound technology, which are applied in the directions of silicon organic compounds, chemical instruments and methods, compounds of elements of Group 4/14 of the periodic table, etc., to achieve the effects of easy products, simple steps, and non-toxic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0043] Example 1
[0044] The synthetic method of naphthosilole compound, comprises the following steps:
[0045] In a sealed tube, add o-alkylarylvinylsilane A1 (26.2 mg, 0.1 mmol), 4-methylbenzenesulfonyl azide (59.1 mg, 0.3 mmol), [Ir{dt(tBu) 2 ppy} 2 (dtbbpy)][PF 6 ] (0.6mg, 0.0005mmol), 1,4-diazabicyclo[2.2.2]octane (triethylenediamine) (16.8mg, 0.15mmol), 1,4-dioxane (1mL) The mixed solution was irradiated by 2×30W blue LEDs, stirred at 80°C, and reacted for 24h, then stopped heating, cooled to room temperature, spin-dried, and further separated and purified by column chromatography (eluent was petroleum ether) to obtain 24.0 mg of the product, Yield: 69%.
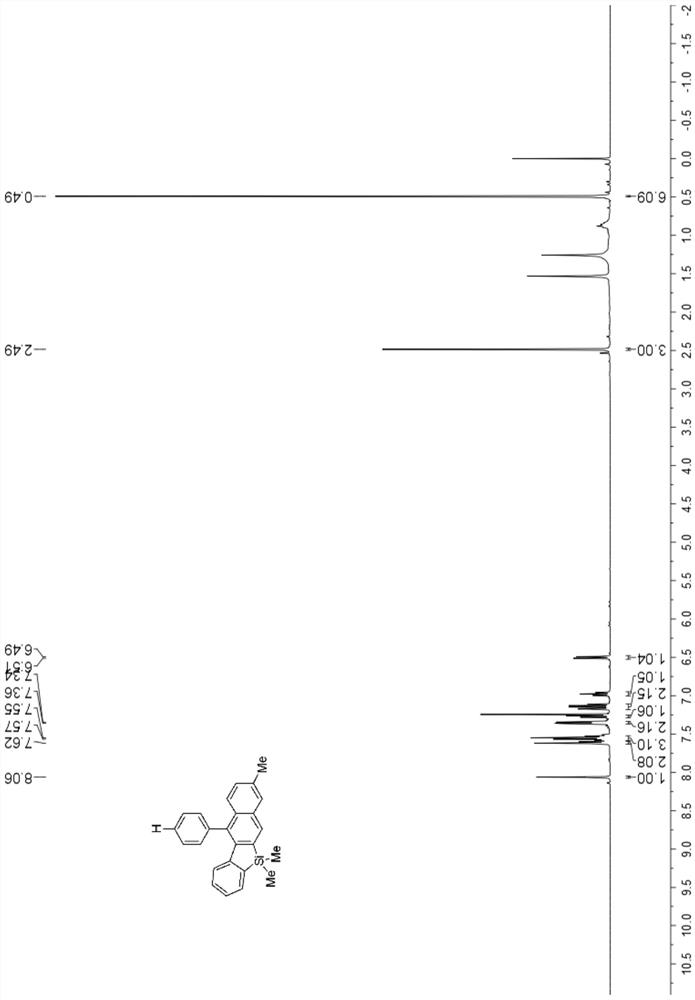
[0046] The structural characterization data of the product obtained in this embodiment are as follows:
[0047] 1 H NMR (400MHz, CDCl 3 )δ8.06(s,1H),7.62–7.60(m,2H),7.58–7.52(m,3H),7.36–7.34(m,2H),7.27(d,J=8.7Hz,1H),7.17 –7.11 (m, 2H), 7.00 – 6.96 (m, 1H), 6.50 (d, J=8.2Hz, 1H), 2.49 (s, 3H), 0.49 (s, 6H). figu
Example Embodiment
[0052] Example 2
[0053] The synthetic method of naphthosilole compound, comprises the following steps:
[0054] In a sealed tube, add o-alkylarylvinylsilane A1 (26.2 mg, 0.1 mmol), 4-phenylbenzenesulfonyl azide (77.7 mg, 0.3 mmol), [Ir{dt(tBu) 2 ppy} 2 (dtbbpy)][PF 6 ] (0.6mg, 0.0005mmol), 1,4-diazabicyclo[2.2.2]octane (16.8mg, 0.15mmol), 1,4-dioxane (1mL); Irradiated by 30W blueLEDs, stirred at 80°C, reacted for 24 hours, stopped heating, cooled to room temperature, spin-dried, and further separated and purified by column chromatography (petroleum ether as the eluent) to obtain 18.0 mg of the product with a yield of 43%.
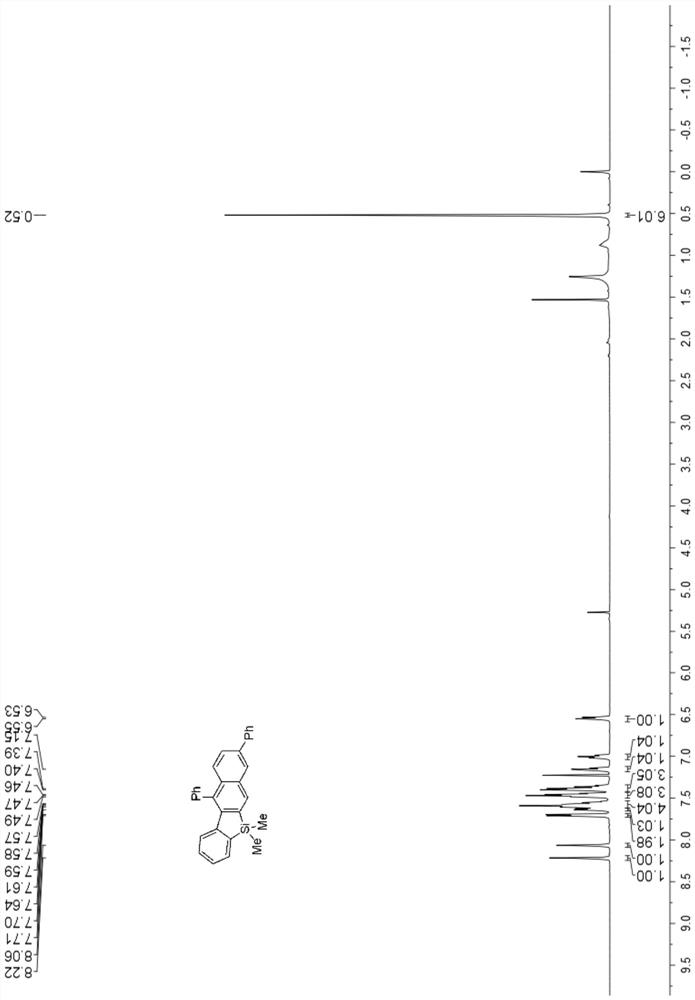
[0055] The structural characterization data of the product obtained in this embodiment are as follows:
[0056] 1 H NMR (500MHz, CDCl 3 )δ8.22(s,1H),8.06(s,1H),7.70(d,J=7.2Hz,2H),7.63(d,J=6.6Hz,1H),7.61–7.52(m,4H), 7.46(dd, J=13.6,7.6Hz,3H),7.38(dd,J=17.7,7.0Hz,3H),7.15(t,J=7.0Hz,1H),7.00(t,J=7.2Hz,1H ), 6.54(d, J=8.0Hz, 1H), 0.52(s, 6H). image 3 .
Example Embodiment
[0061] Example 3
[0062] The synthetic method of naphthosilole compound, comprises the following steps:
[0063] In a sealed tube, add o-alkylarylvinylsilane A1 (26.2 mg, 0.1 mmol), 4-cyanobenzenesulfonyl azide (62.4 mg, 0.3 mmol), [Ir{dt(tBu) 2 ppy} 2 (dtbbpy)][PF 6 ] (0.6mg, 0.0005mmol), 1,4-diazabicyclo[2.2.2]octane (16.8mg, 0.15mmol), 1,4-dioxane (1mL); Irradiated by 30W blueLEDs, stirred at 80°C, reacted for 24 hours, stopped heating, cooled to room temperature, spin-dried, and further separated and purified by column chromatography (petroleum ether as the eluent) to obtain 26.0 mg of the product with a yield of 72%.
[0064] The structural characterization data of the product obtained in this embodiment are as follows:
[0065] 1 H NMR (400MHz, CDCl 3 )δ8.27(s,1H),8.23(s,1H),7.70(d,J=7.0Hz,1H),7.65–7.63(m,3H),7.49(s,2H),7.39–7.37(m ,2H),7.25(t,J=7.2Hz,1H),7.06(t,J=7.6Hz,1H),6.59(d,J=8.2Hz,1H),0.57(s,6H). See Figure 5 .
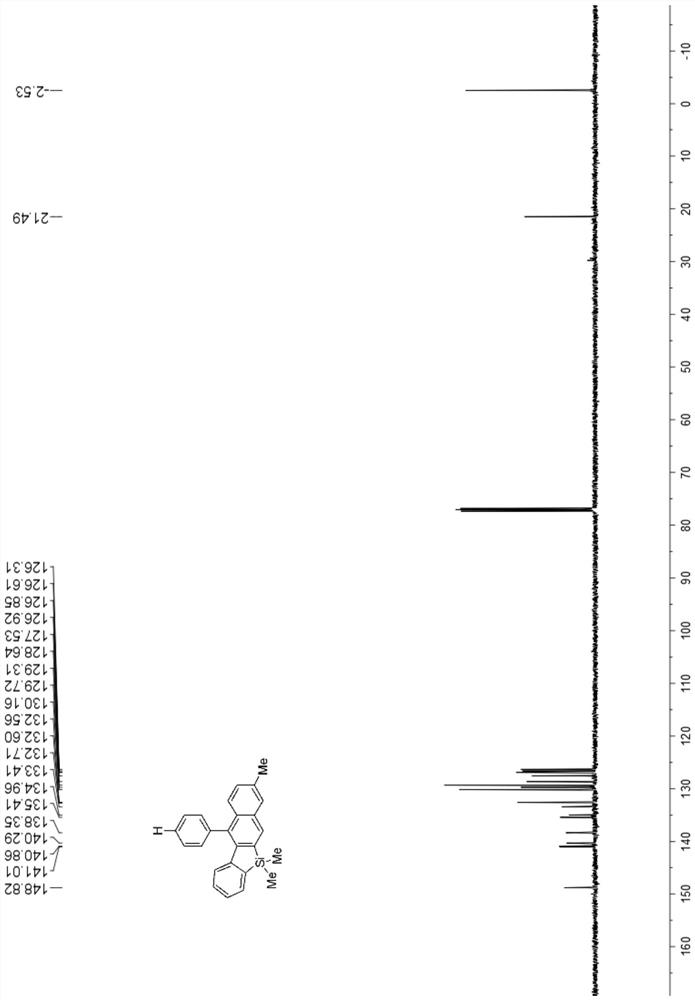
[0066] 13 C NMR (101MHz, CDCl 3 )δ147.7
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap