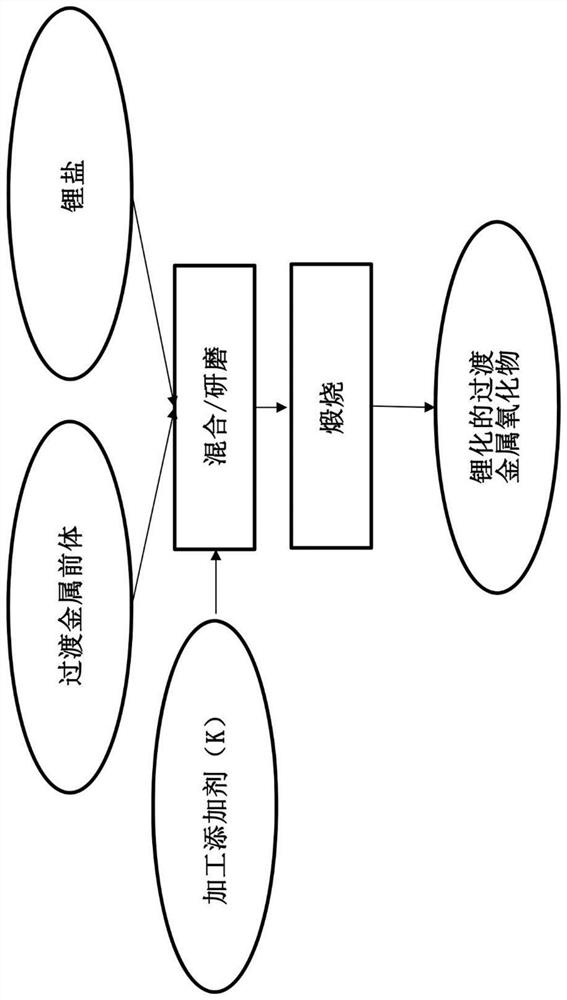
A process for producing lithiated transition metal oxides
A technology of transition metals and oxides, applied in chemical instruments and methods, nickel compounds, chemical/physical processes, etc., can solve the problem that the battery cell does not realize the full theoretical capacity of the active material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The active material precursor is formed using refined nickel hydroxide. 6.7 g of nickel hydroxide (as described in U.S. Patent Nos. 6,432,580 and 6,444,363), 3.35 g of lithium hydroxide (LiOH*H 2 O) and 0.1 g of K 2 CO 3 (2 wt% (relative to nickel hydroxide)). The material was dry mixed to homogeneity using a SPEX CETRIPREP 8000 mixer / mill for 20 minutes. As a control, without including K 2 CO 3 In the case of forming compositionally identical materials.
[0054] Make the resulting active material precursor flow O at 885° C. 2 in calcination. Calcination was carried out for 15 hours. Some of the resulting calcined granules were manually crushed using a mortar and pestle and optionally precharged by standard techniques for downstream electrochemical analysis.
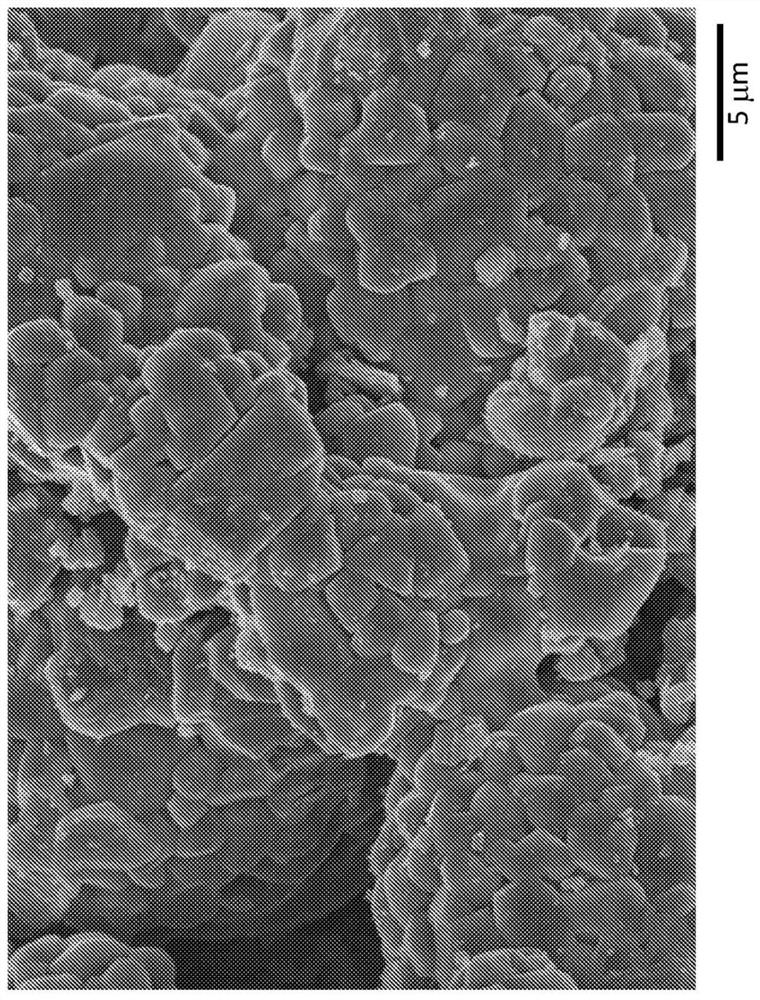
[0055] In the absence or presence of K 2 CO 3 The particle topology of the material formed in the case of , where the corresponding micrographs were as Figure 2A with 2B as shown. Primary particles i
Embodiment 2
[0060] By adding 1.4 g of LiOH and 0.1 g of K 2 CO 3 (2wt% (relative to the mixed metal hydroxides)) with the addition of 5 g of the precursor Ni 0.8 co 0.1 mn 0.1 (OH) 2 To prepare lithiated cathode material LiNi 0.8 co 0.1 mn 0.1 o 2 . The control material was formed in the same manner, but in the absence of K 2 CO 3 in the case of. The material was mechanically mixed with a SPEX CETRIPREP 8000 mixer / mill for 20 minutes. The resulting powdery mixture was then sintered at 850° C. for 15 hours. The resulting lithiated composite was then cooled to 25°C.
[0061] In the absence or presence of K 2 CO 3 The particle topology of the NCM 811 material formed in the case of the corresponding micrographs were as Figure 3A with 3B as shown. The relative particle sizes are shown in Table 2.
[0062] Table 2: In the presence or absence of K 2 CO 3 The average particle size of the manufactured NCM material in the case.
[0063]
[0064] For electrochemical analysis
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap