Method for detecting related substances of 2, 3-dimethyl bromobenzene
A technology of dimethylbromobenzene and related substances, which is applied in the field of analytical chemistry, can solve problems affecting the quality of medicines and the health of patients, and achieve the effects of short detection time, high detection sensitivity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0030] Example 1:
[0031] A method of detection of 2,3-dimethylphenylphenylphenylphenyl, specifically includes the steps of:
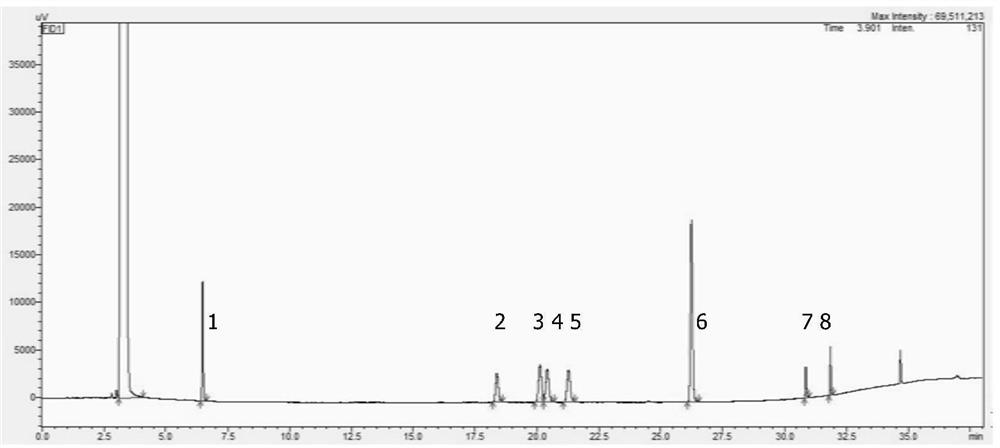
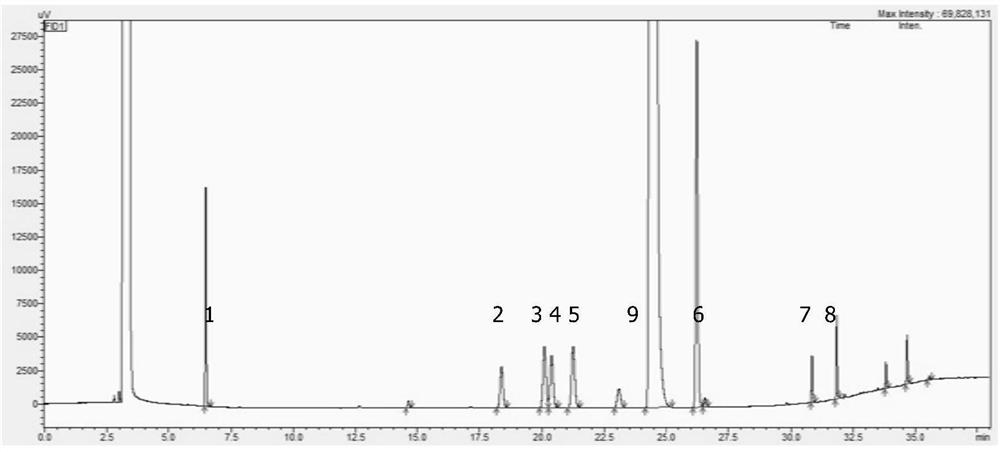
[0032] Take acetonitrile as a white solvent; take the right amount of 2,3-dimethyl bromobenzene, dissolve with acetonitrile, and produce 10 mg / ml of 2,3-dimethyl bromobenzene solution, as the test solution; Xylene, 3-nitroenen xylene, 2,3-dimethylennemine, 4-bromo-1,2-xylene, 2-bromo-1,4-xylene, 2,6-dimethyl bromide Benzene, 2,4-dimethyl bromobenzene and 5-bromoate, adding acetonitrile, prepared to prepare 20 μg, 3-nitroxyl xylene 10 μg, 2,3-dimethylaniline per 1 ml 10 μg, 4-bromo-1,2-xylene 100 μg, 2-bromo-1,4-xylene 30 μg, 2,6-dimethyl bromobenzene 30 μg, 2,4-dimethyl bromobenzene 30 μg and 5- A solution of a 20 μg of xylene between bromine as a control solution;
[0033] The chromatographic conditions of gas chromatography are:
[0034] Chromatograph: Japan Island GC2030;
[0035] Columns: 2,6-di -O-pentyl-3-methoxy derivative modified β-cyclodextrin
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Column length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap