Preparation method of hexafluorodianhydride
A technology of hexafluorodianhydride and hexafluorotetraic acid, which is applied in the field of preparation of fluorine-containing monomers, can solve the problems of easy explosion, high requirements for reaction vessels, low safety, etc., and achieves high safety and good reaction effect. , the effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
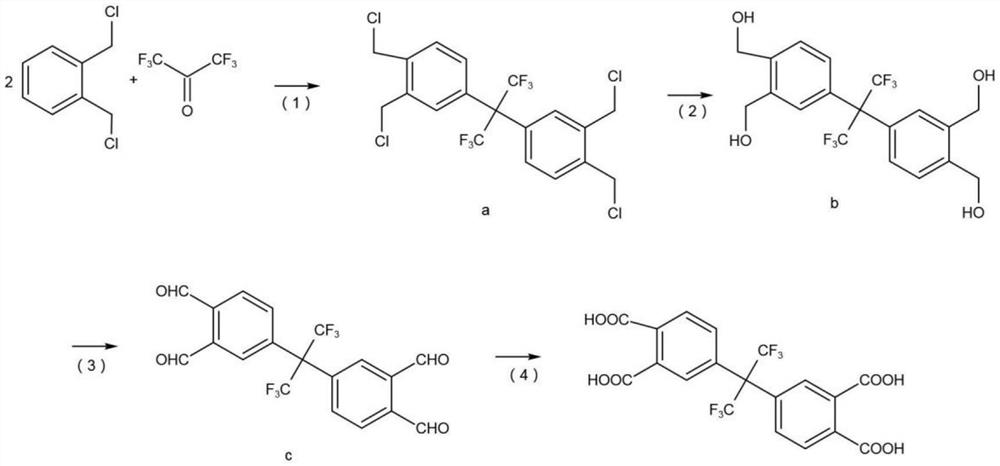
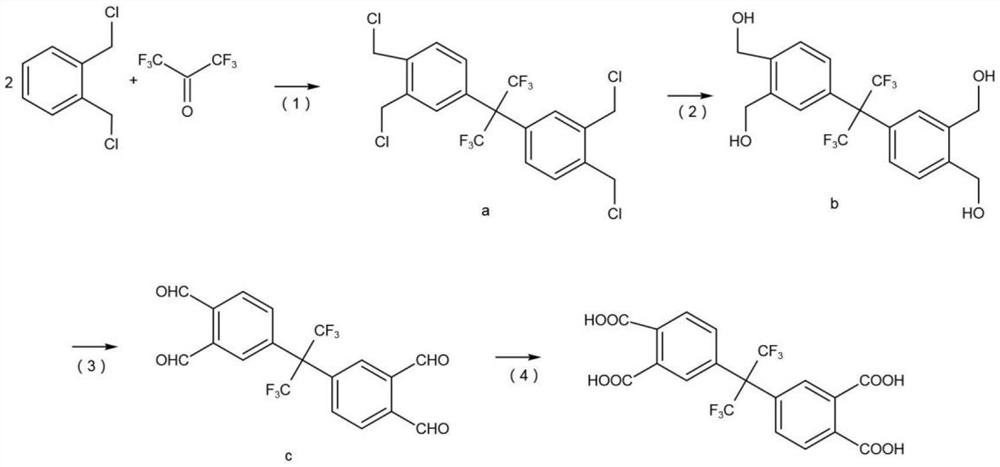
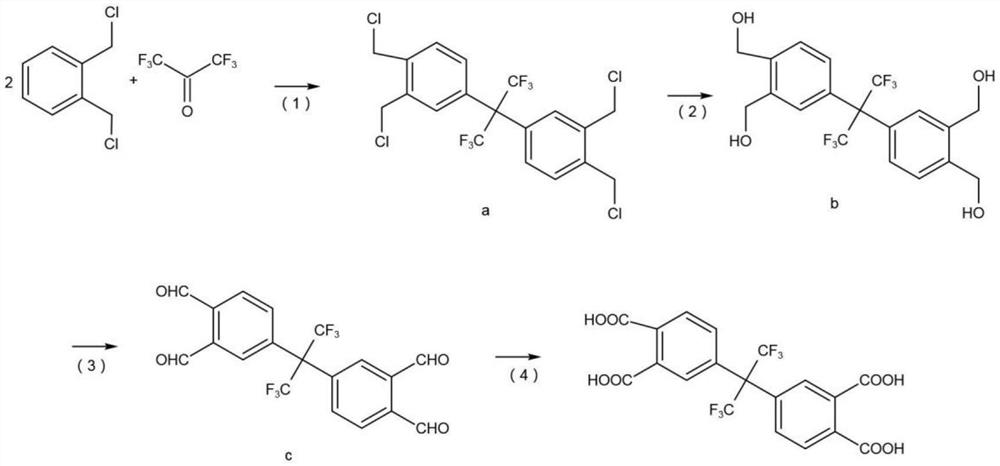
[0033] A preparation method of hexafluorodianhydride, comprising the following steps:
[0034] S1, prepare hexafluorotetraacid, the reaction equation is as follows:
[0035]
[0036] (1) Add 0.2mol o-dichlorobenzyl to a 500L flask, add 40ml concentrated sulfuric acid, pass through nitrogen protection, stir at 130°C, gradually add 0.2mol hexafluoroacetone trihydrate as raw material, reflux for 20h, after the reaction The product was washed with water, washed with alkali, and filtered to obtain product a with a yield of 94.1%.
[0037] (2) product a is added in the aqueous solution of soda ash and carries out hydrolysis reaction, and hydrolysis reaction uses 4g tetrapropyl ammonium bromide as phase transfer catalyst and 10g dimethylbenzene as solvent, starts to stir, and the mol ratio of product a and sodium bicarbonate and water is 1:2.2:3.0, the reaction temperature is 140°C, the reaction time is 6h, and the product b is obtained after distillation with a yield of 95.4%.
[
Embodiment 2
[0046] A kind of preparation method of hexafluorodianhydride, the difference with embodiment 1 is:
[0047] In step (1) of step S1, the reaction temperature is 140° C., and the reflux time is 30 h. The output of the product a of step (1) in the S1 step is 23.50 g, and the yield is 94.4%.
Embodiment 3
[0049] A kind of preparation method of hexafluorodianhydride, the difference with embodiment 1 is:
[0050]In step (1) of step S1, the reaction temperature is 150° C., and the reflux time is 40 h. The yield of the product a of step (1) in the S1 step is 94.2%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap