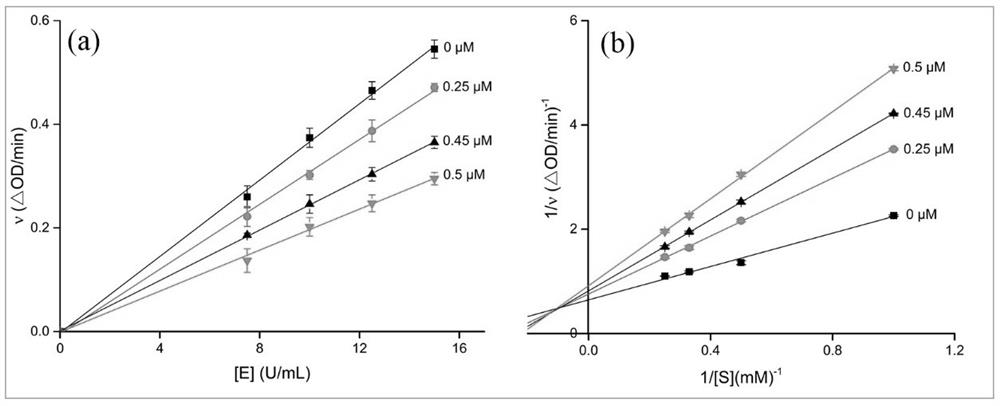
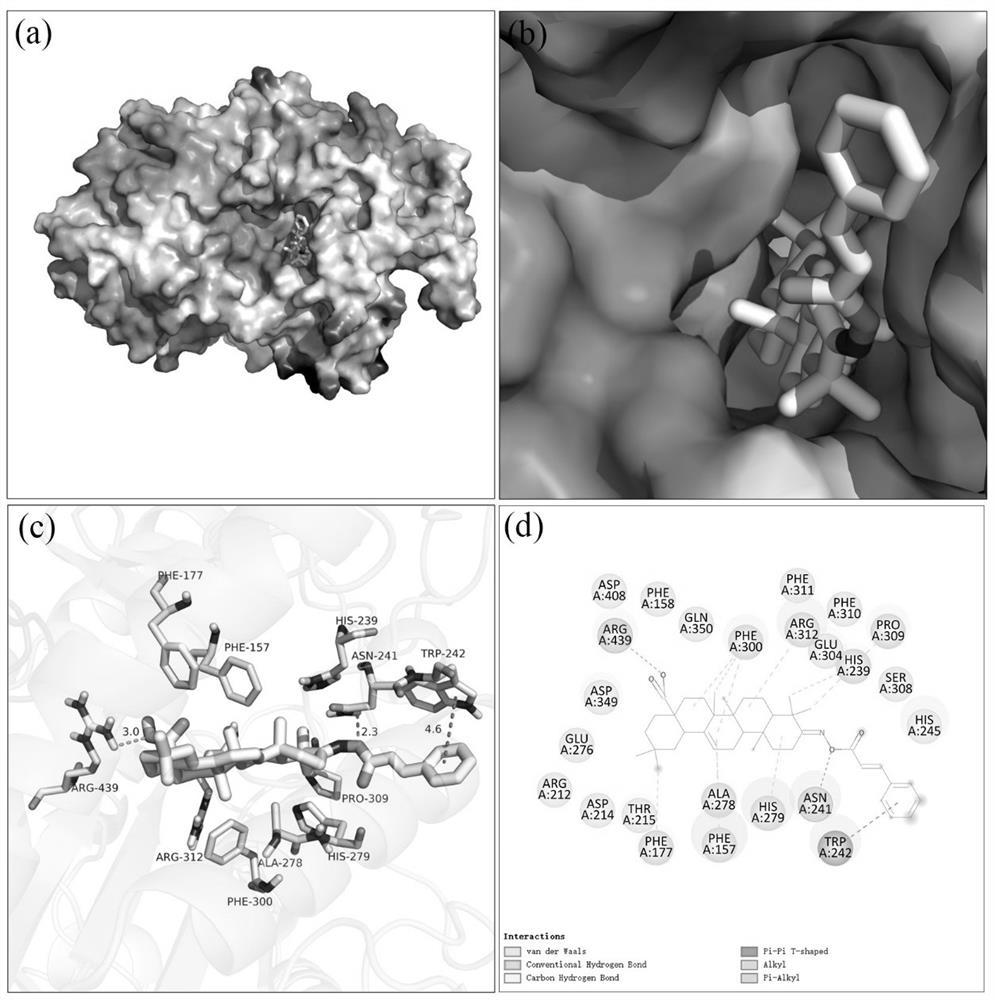
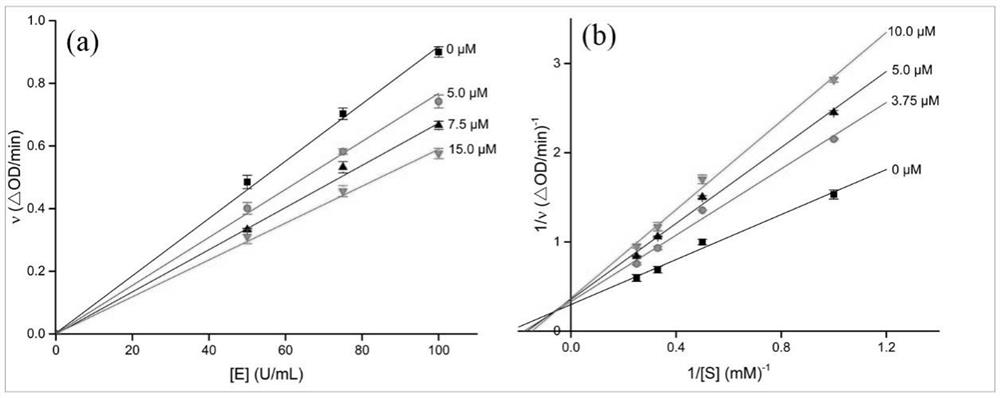
Oleanolic acid oxime ester derivative and preparation method and application thereof
A technology for oleanolic acid oxime and derivatives, which is applied in the field of biomedicine, can solve the problems of inability to meet clinical application, low α-glucosidase and α-amylase inhibitory effects, and achieves the effect of good application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation of oleanolic acid oxime ester derivative 3a and its application in inhibiting the activity of α-glucosidase. The structural formula of oleanolic acid oxime ester derivative 3a is as follows:
[0053]
[0054] Oleanolic acid oxime ester derivative 3a is prepared by the following steps:
[0055] (1) Place the solution of oleanolic acid (1.0 mmol) in acetone (10 mL) in an ice bath, and add freshly prepared Jones reagent dropwise. The reaction mixture was brought to room temperature and stirred for 4 hours. Methanol and water were added to the reaction mixture in turn, concentrated to remove the organic solvent, extracted with ethyl acetate, washed with saturated brine, dried over anhydrous magnesium sulfate, concentrated, separated and purified by column chromatography to obtain compound 1.
[0056] (2) Subsequently, to NH 2 Add NaOAc (2.0 mmol) to a solution of OH·HCl (1.5 mmol) in ethanol (10 mL), and stir at room temperature for 15 minutes. Compound 1 was
Embodiment 2
[0065] Preparation of oleanolic acid oxime ester derivative 3f and its application in inhibiting the activity of α-amylase. The structural formula of oleanolic acid oxime ester derivative 3f is as follows:
[0066]
[0067] Oleanolic acid oxime ester derivative 3f is obtained by the similar operation steps as oleanolic acid oxime ester derivative 3a, wherein in step (3), to the DCM solution of compound 2 and pyridine, add newly prepared (E) -3-(4-Bromophenyl)acryloyl chloride. Oleanolic acid oxime ester derivative 3f was a white solid with a yield of 42%.
[0068] Characterize the structure of the prepared compound to verify the correctness of its structure. 1 H NMR (500 MHz, CDCl 3 ) δ 7.70 (d, J = 16.0 Hz, 1H), 7.53 (dd, J = 8.7, 2.4 Hz, 2H), 7.46 -7.39 (m, 2H), 6.56 (d, J = 16.0 Hz, 1H), 5.29 (d, J = 3.6 Hz, 1H), 3.01 (dt, J = 15.1, 4.6 Hz, 1H), 2.82 (dd, J = 14.3, 4.9 Hz, 1H), 2.37 (ddd, J = 14.8,12.2, 5.8 Hz, 1H), 2.03 - 1.91 (m, 2H), 1.86 (ddd, J = 18.4
Embodiment 3
[0075] The structural characterization of other derivatives in the technical solution of the present invention is as follows, prepared with reference to the preparation method of Example 1, and verified by experiments that it is the same as Examples 1 and 2, and has inhibitory effects on both α-glucosidase and α-amylase.
[0076]
[0077] Characterize the structure of the prepared compound to verify the correctness of its structure. White solid, yield 60%. 1 HNMR (500 MHz, CDCl 3 ) δ 7.75 (d, J = 16.0 Hz, 1H), 7.45 (d, J = 7.9 Hz, 2H),7.20 (d, J = 7.8 Hz, 2H), 6.53 (d, J = 16.0 Hz, 1H), 5.29 (t, J = 3.7 Hz,1H), 3.03 (dt, J = 15.2, 4.5 Hz, 1H), 2.82 (dd, J = 14.0, 4.6 Hz, 1H), 2.38(s, 4H), 2.42 - 2.32 (m, 1H), 2.03 - 1.82 (m, 4H), 1.82 - 1.72 (m, 2H), 1.72- 1.55 (m, 5H ), 1.47 (ddt, J = 18.4, 13.5, 7.8 Hz, 2H), 1.35 (ddd, J = 13.0,9.0, 3.2 Hz, 3H), 1.30 - 1.21 (m, 2H), 1.21 - 1.07 (m, 9H), 1.05 (s, 3H),0.99 - 0.85 (m, 7H), 0.80 (s, 3H ). 13 C NMR (125 MHz,
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap