Sucralose-6-ester purification method
A technology of sucralose and preset conditions, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problem of low yield of sucralose-6-ethyl ester, difficulty in subsequent waste liquid treatment, separation of Low efficiency and other problems, to achieve the effect of reducing impurity content, strong practicability and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
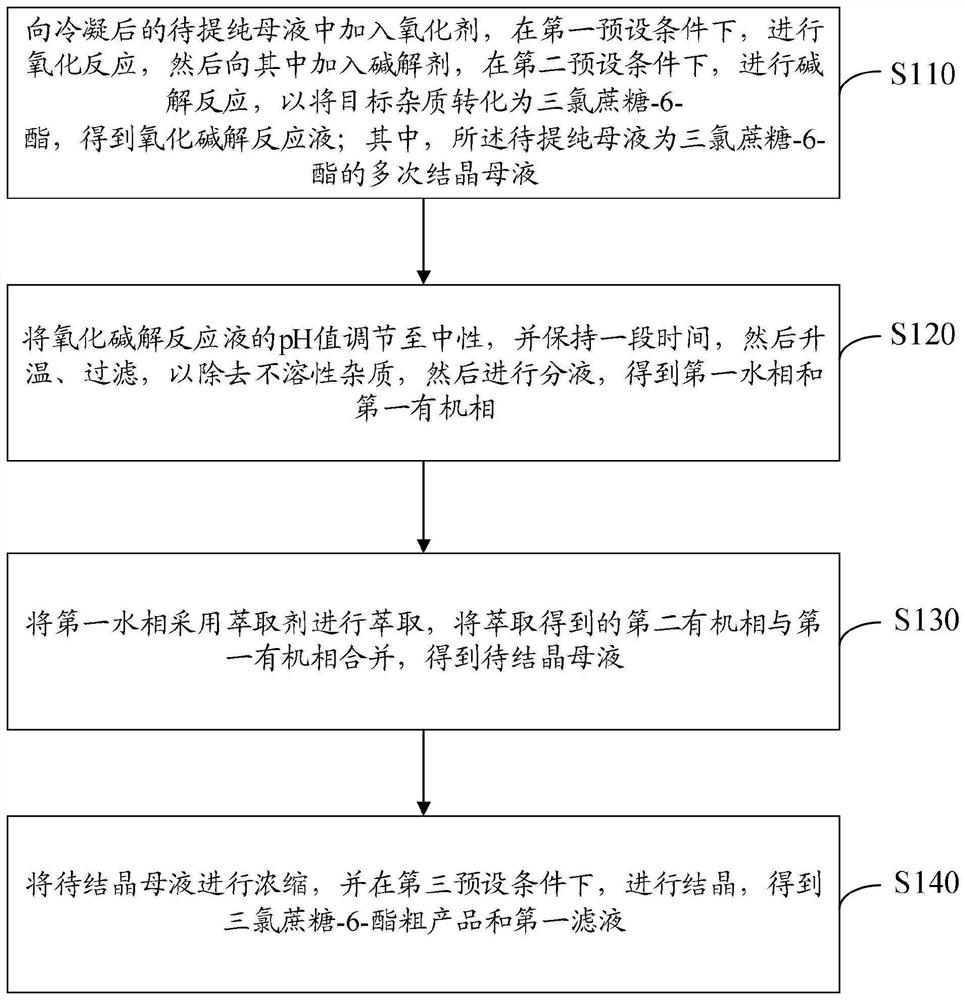
[0090] a. Take 1000mL of the secondary mother liquor of sucralose-6-acetate (the initial concentration of sucralose-6-acetate is 32g / L) and add it into a 2000mL three-necked flask, and cool down to 2±2°C. Hydrogen peroxide with a mass fraction of 27.5% was added dropwise to carry out the oxidation reaction. The amount of hydrogen peroxide was 120 mL. After the hydrogen peroxide was added dropwise, it was incubated for 6 hours, and the temperature was maintained at about 5°C.
[0091] b. After the reaction of the materials in step a, add 125mL of dimethylamine with a mass fraction of 40% to carry out the alkali hydrolysis reaction. The reaction temperature is controlled at about 5°C, and the pH of the reaction is controlled at about pH=9. After the pH is stable, keep warm and maintain the alkali hydrolysis The time is controlled at about 10 hours.
[0092] c. Add hydrochloric acid with a mass fraction of 30% to the reaction solution in step b above, and adjust its pH value to ab
Embodiment 2
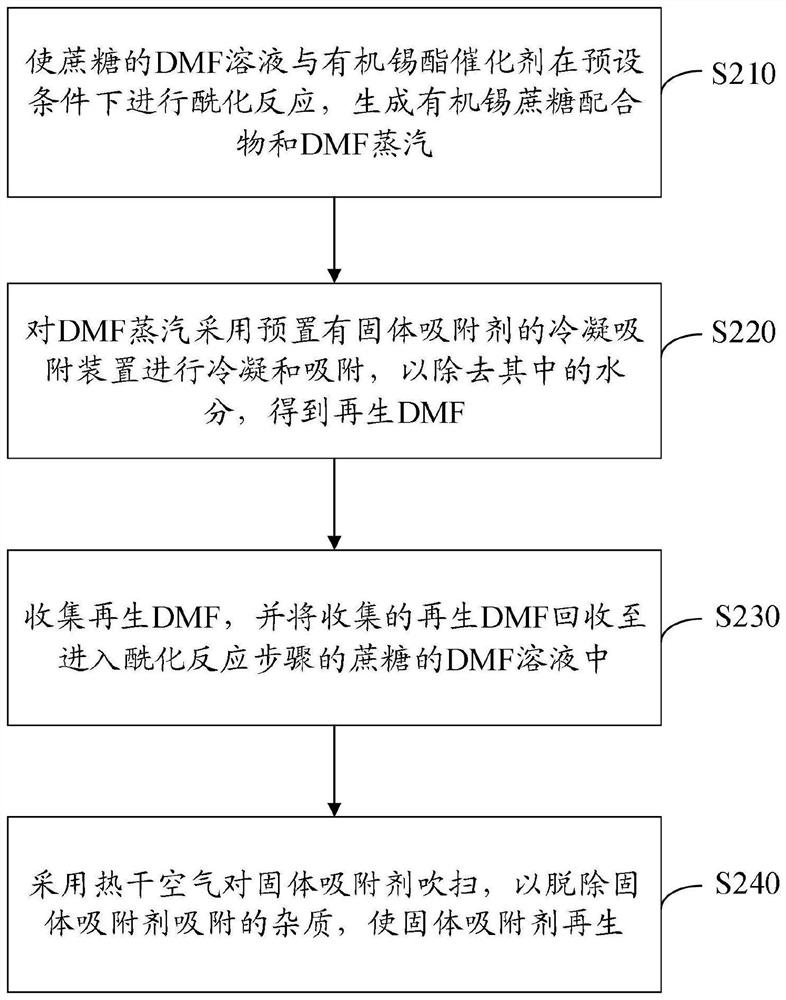
[0096] a. Take 1000mL of the secondary mother liquor of sucralose-6-acetate (the initial concentration of sucralose-6-acetate is 32g / L) and add it into a 2000mL three-necked flask, and cool down to 2±2°C. Hydrogen peroxide with a mass fraction of 27.5% was added dropwise to carry out the oxidation reaction. The amount of hydrogen peroxide was 120 mL. After the hydrogen peroxide was added dropwise, it was incubated for 6 hours, and the temperature was maintained at about 5°C.
[0097] b. After the reaction of the materials in step a, add 125mL of dimethylamine with a mass fraction of 40% to carry out the alkali hydrolysis reaction. The reaction temperature is controlled at about 5°C, and the pH of the reaction is controlled at about pH=9. After the pH is stable, keep warm and maintain the alkali hydrolysis The time is controlled at about 10 hours.
[0098] c. Add hydrochloric acid with a mass fraction of 30% to the reaction solution in step b above, and adjust its pH value to ab
Embodiment 3
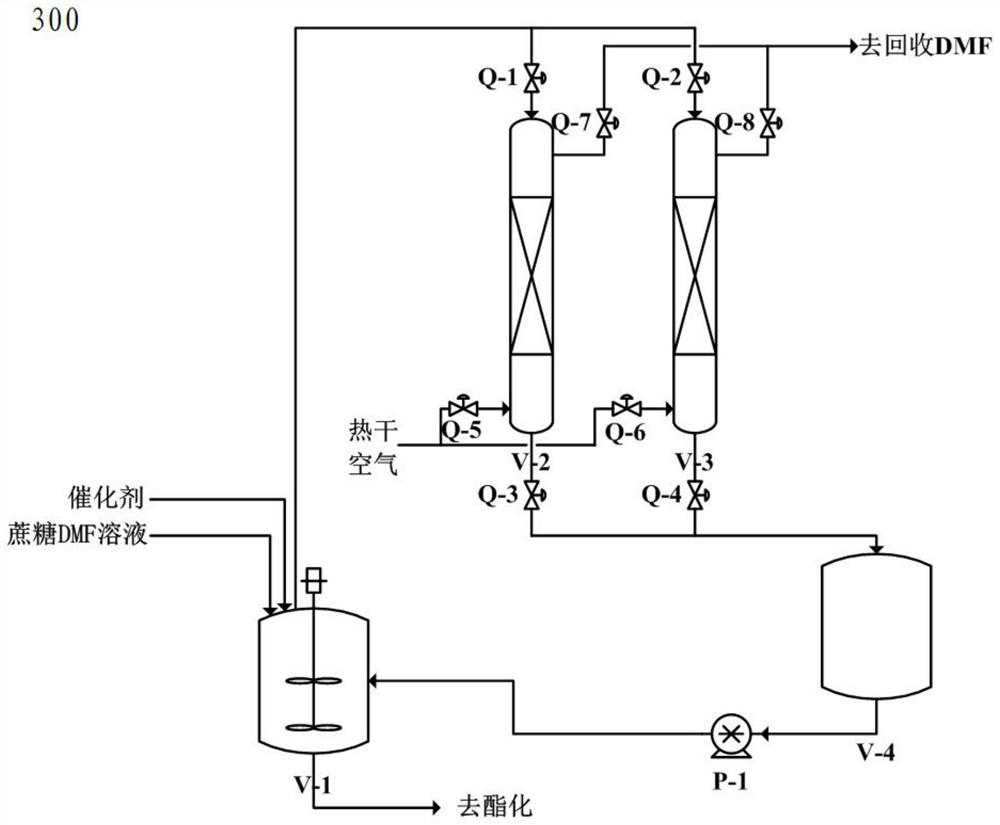
[0103] a. Take 1000 mL of the secondary mother liquor of sucralose-6-acetate (the initial concentration of sucralose-6-acetate is 32 g / L) and add it into a 2000 mL three-necked flask, and cool down to -10°C. Hydrogen peroxide with a mass fraction of 40% was added dropwise to carry out the oxidation reaction. The amount of hydrogen peroxide was 50 mL. After the hydrogen peroxide was added dropwise, the temperature was kept at about 5°C for 12 hours.
[0104] b. After the reaction of the materials in step a, add 150mL of dimethylamine with a mass fraction of 30% to carry out the alkali hydrolysis reaction. The reaction temperature is controlled at about 5°C, and the pH of the reaction is controlled at about pH=9. After the pH is stable, keep warm and maintain the alkali hydrolysis The time is controlled at about 12 hours.
[0105] c. Add hydrochloric acid with a mass fraction of 15% to the reaction solution in the above step b, and adjust its pH value to about pH=7.0. The amount
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap