Method for culturing cell sphere by using self-assembled polypeptide derivative hydrogel, cell sphere and application of cell sphere
A technology for peptide derivatives and cell culture, applied in biochemical equipment and methods, epidermal cells/skin cells, tissue culture, etc., can solve the problems of low cell activity and complex preparation process of cell spheroids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Weigh Biotin- D 10 mg of F-YIGSR polypeptide derivative powder was placed in a glass vial, and 994.84 μL of 1×PBS (pH=7.4) and 5.16 μL of 1M Na were added 2 CO 3 solution, and mix well. Heat the vial with an alcohol lamp until the solution boils, then let it stand still, and after cooling to room temperature, the self-assembled polypeptide derivative hydrogel is obtained.
Embodiment 2
[0053] Take 37.5 μL of the self-assembled polypeptide derivative hydrogel obtained in Example 1, vortex and mix evenly with 12.5 μL of MCF-7 cell suspension containing 3000 cells, transfer to a 96-well plate, and put it into a cell culture incubator. After the cell gel mixture was placed in the incubator for 10 min, 100 μL of DDMEM (10% FBS) medium was added to its surface, and 50 μL of fresh complete medium was replaced every other day.
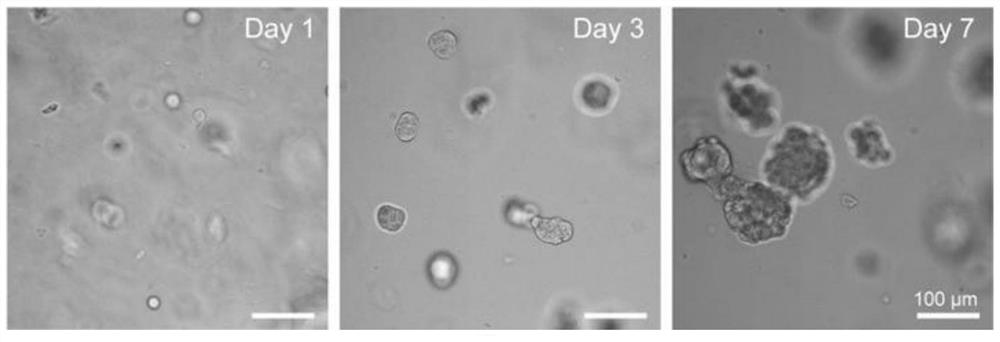
[0054] Record the growth of the cell spheroids, the results are as follows: figure 1 shown.
[0055] The results showed that the cell spheres could form normally and gradually increase in the 1-7 days; on the 7th day, the average cross-sectional area of the cell spheres formed by MCF-7 was 4610 μm 2 .
Embodiment 3
[0057] Take 37.5 μL of the self-assembled polypeptide derivative hydrogel obtained in Example 1, vortex and mix evenly with 12.5 μL of 4T1 cell suspension containing 3000 cells, transfer to a 96-well plate, and put it into a cell culture incubator. After the cell gel mixture was placed in the incubator for 12 minutes, 100 μL of RPMI1640 (10% FBS) medium was added to its surface, and 50 μL of fresh complete medium was replaced every other day.
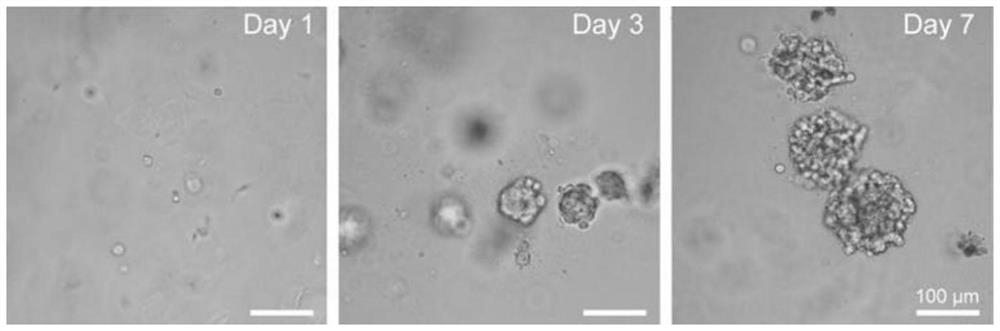
[0058] Record the growth of the cell spheroids, the results are as follows: figure 2 shown.
[0059] The results showed that the cell spheres could form normally and gradually increase in the 1-7 days; on the 7th day, the average cross-sectional area of the cell spheres formed by 4T1 was 11450 μm 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average cross-sectional area | aaaaa | aaaaa |
| Average cross-sectional area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap