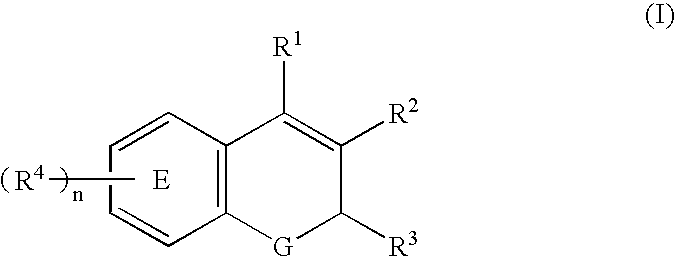
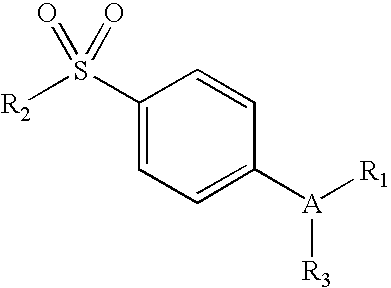
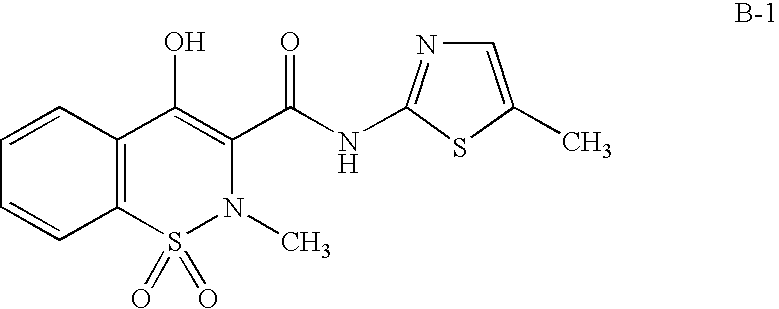
Mediated central nervous system compositions of a cyclooxygenase-2 selective inhibitor and a corticotropin releasing factor antagonist for the treatment of ischemic disorders or injury
a technology of cyclooxygenase and central nervous system, which is applied in the direction of biocide, heterocyclic compound active ingredients, amide active ingredients, etc., can solve the problems of brain or spinal cells losing their ability to produce energy, brain or spinal cells becoming damaged, and affecting the severity of stroke patients, so as to improve the severity of stroke symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Evaluation of COX-1 and COX-2 Activity in Vitro
[0473] The COX-2 inhibitors suitable for use in this invention exhibit selective inhibition of COX-1 over COX-2, as measured by IC50 values when tested in vitro according to the following activity assays.
Preparation of Recombinant COX Baculoviruses
[0474] Recombinant COX-1 and COX-2 are prepared as described by Gierse et al, [J. Biochem., 305, 479-84 (1995)]. A 2.0 kb fragment containing the coding region of either human or murine COX-1 or human or murine COX-2 is cloned into a BamH1 site of the baculovirus transfer vector pVL1393 (Invitrogen) to generate the baculovirus transfer vectors for COX-1 and COX-2 in a manner similar to the method of D. R. O'Reilly et al (Baculovirus Expression Vectors: A Laboratory Manual (1992)). Recombinant baculoviruses are isolated by transfecting 4 μg of baculovirus transfer vector DNA into SF9 insect cells (2×108) along with 200 ng of linearized baculovirus plasmid DNA by the calcium phosphate method. S
example 2
[0480] In the examples below, a combination therapy contains a corticotropin releasing factor antagonist with a vehicle and a COX-2 selective inhibitor. The efficacy of such combination therapy can be evaluated in comparison to a control treatment such as a placebo treatment, administration of a COX-2 inhibitor only, or administration of a corticotropin releasing factor antagonist only. By way of example, a combination therapy may contain α-helical CRF 9-41 and celecoxib, α-helical CRF 9-41 and valdecoxib, α-helical CRF 9-41 and rofecoxib, or α-helical CRF 9-41 and paracoxib. In addition, any of the above mentioned COX-2 selective inhibitors may be similarly combined with other CRF antagonists, both peptide and non-peptide, such as for example, the pyrrolopyrimidines disclosed in WO 94 / 13676, CP-154,526 (Schulz et. al., Proc. Natl. Acad. Sci. USA, Vol. 93 (1996) 10477-10482), [D-phe12,Nle21,38]hCRF-(12-14) (Gulyas et al., Proc. Natl. Acad. Sci. USA, Vol 92 (1995) 10575-10579), D-phe
example 3
[0486] This laboratory animal study can generally be performed as described in Lyons, et al., (1991) Brain Research, 545: 339-342.
[0487] Male Winstar rats weighing 250-300 g are induced with 2.0% halothane, intubates, and ventilated with 1.0% halothane. PE-50 catheters are inserted into the femoral vein and artery, Core body temperature is monitored and maintained at 37.0±0.5° C. An intraventricular injection of α-helical CRF 9-41 is administered 0.80 mm posterior, 1.50 mm lateral to the bregma and 3.80 mm deep. Fifteen minutes after injection, transient forebrain ischemia is produced for a 10 minute period by the temporary occlusion of both common carotid arteries and phlebotomizing the rat to a MABP of 50 mm Hg. After 10 minutes of ischemia, previously withdrawn and heparinized blood was infused via the femoral venous catheter and cerebral blood flow is restored by removal of the carotid artery clips. Animals are then monitored under 1.0% halothane for a 1 hour period after reperfus
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dimensionless property | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap