Method for using potassium channel activation for delivering a medicant to an abnormal brain region and/or a malignant tumor
a technology of potassium channel and medicant, applied in the field of medical arts, can solve the problems of limited efficacy of novel therapeutic agents, inability to reach their targets in vivo in adequate quantities, and low permeability to macromolecules and viral particles, so as to reduce the damage to non-malignant tissue, increase the selectivity of drug delivery, and reduce the effect of tumor growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Methods
[0080] Malignant Cell Line and Tumor Implantation. A rat glioma cell line, RG2, was used for implantation of experimental brain tumors in female Wistar rats. The techniques for RG2 cell propagation and maintenance in tissue culture have been described (Sugita, M. and Black, K. L., Cyclic GMP-specific phosphodiesterase inhibition and intracarotid bradykinin infusion enhances permeability into brain tumors, Cancer Res. 58(5): 914-20 [1998]; Inamura et al. [1994]; Nakano, S. et al., Increased brain tumor microvessel permeability after intracarotid bradykinin infusion is mediated by nitric oxide, Cancer Res. 56(17): 4027-31 [1996]). Briefly, RG2 cells derived from a rat glioma are kept frozen until use, then are thawed and maintained in a monolayer culture in F12 medium with 10% calf serum.
[0081] The Wistar rats (approximately 140-160 g body weight) were anesthetized with intra-peritoneal ketamine (50 mg / kg), and glial cells (1×105) were implanted into the right hemisphere, but
example 2
Results
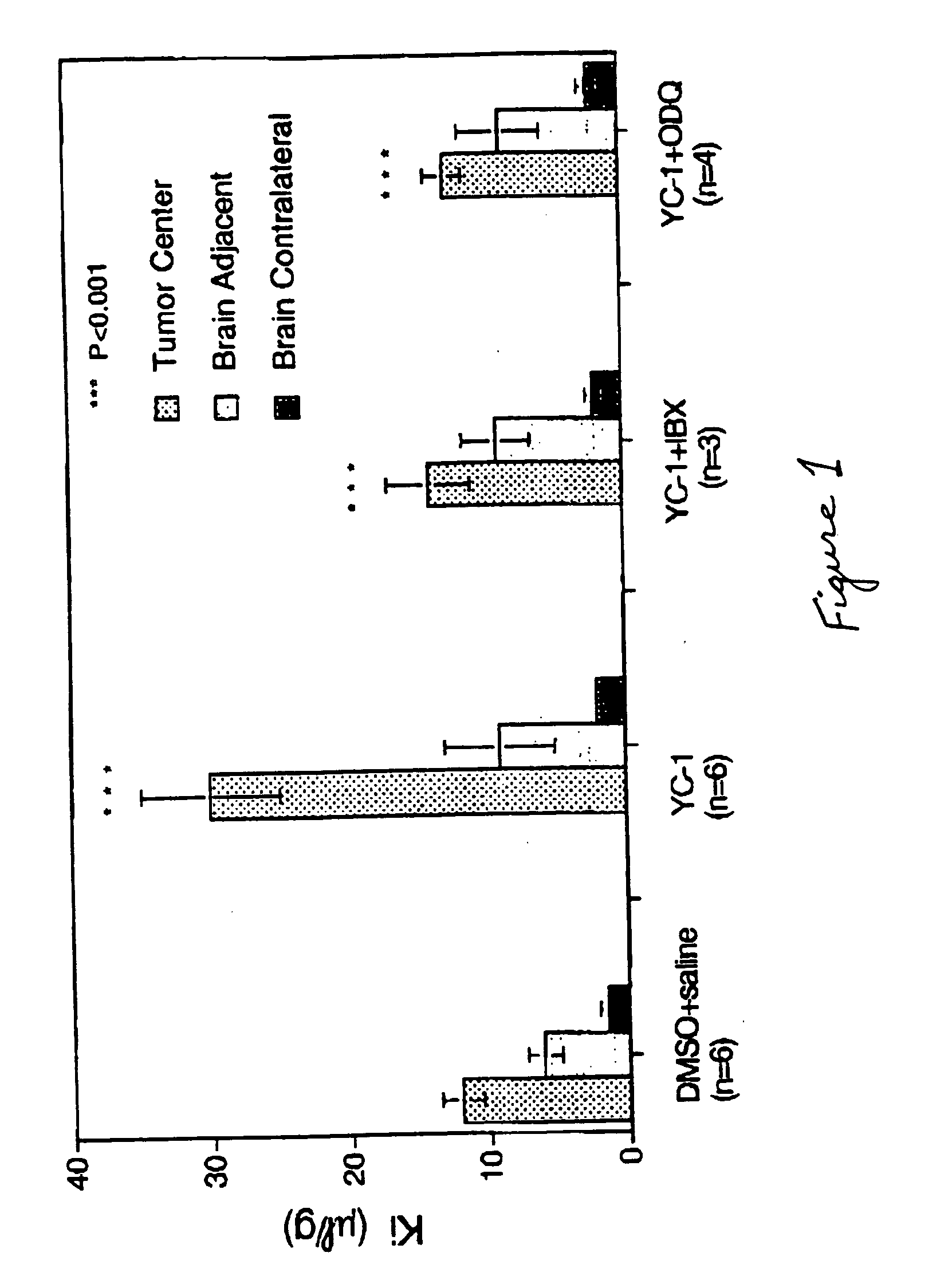
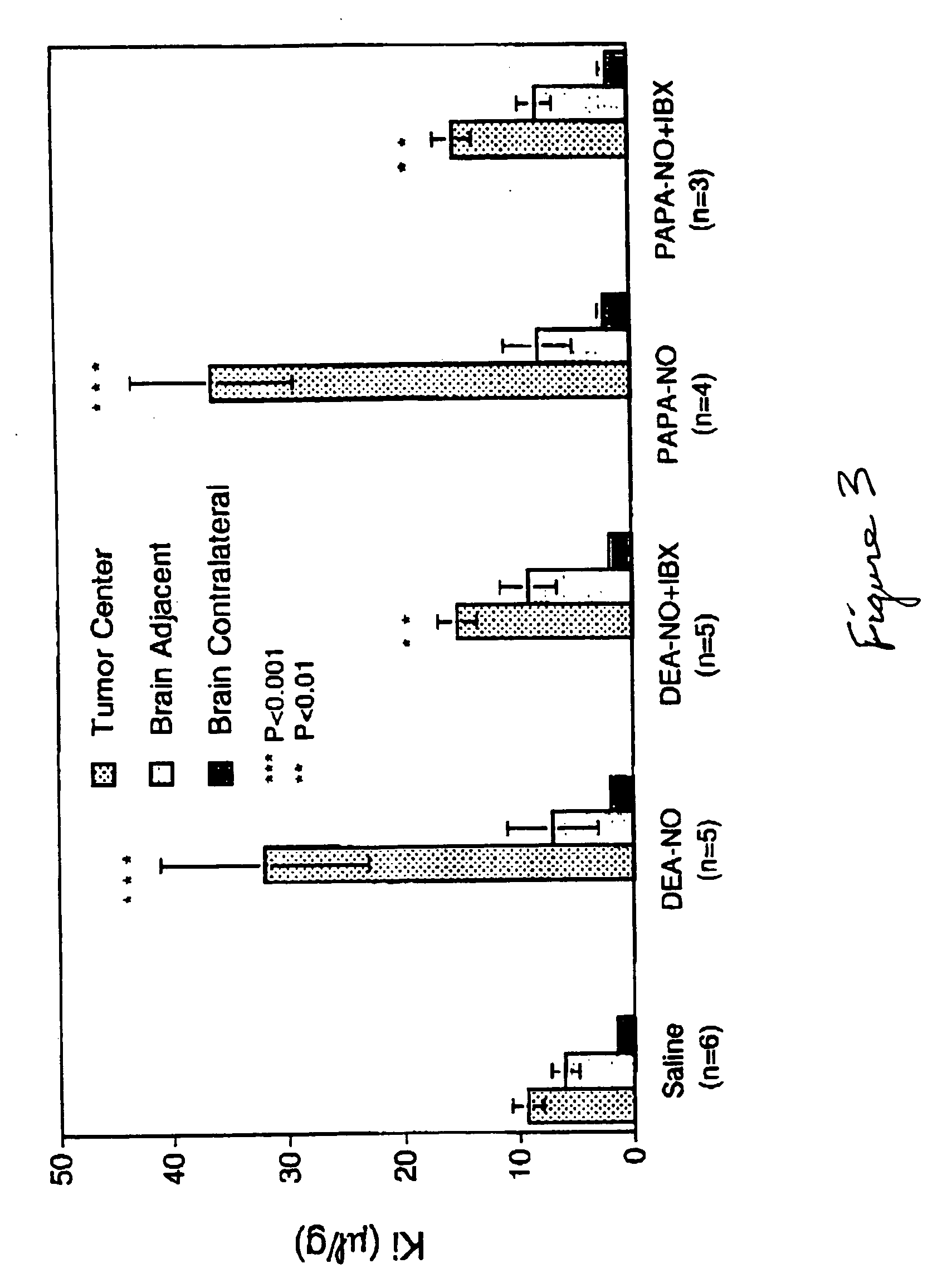
Potassium Channel Activators Selectively Increase Transport Across the Blood-Tumor Barrier.
[0098] When Wistar rats bearing implanted glioma cells were infused with either NS-1619 or minoxidil sulfate, at 7.5 μg kg−1min−1 for 15 minutes, the unidirectional transport constant K for [14C]α-aminoisobutyric acid (AIB) was significantly increased by NS-1619 and minoxidil sulfate with respect to transport across the neovasculature forming the blood-tumor barrier, but not with respect to transport across normal brain microvasculature (data not shown). These results demonstrated that activation of potassium calcium channels selectively increases the permeability of molecules across the capillaries of solid malignant tumors compared to capillaries supplying normal brain tissue.
[0099] Increasing the dose of NS-1619 resulted in an increase in the unidirectional transfer constant Ki for [14C]α-aminoisobutyric acid in RG2 glioma capillaries in a dose-dependent manner (data not shown). At
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dimensionless property | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
| Frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap