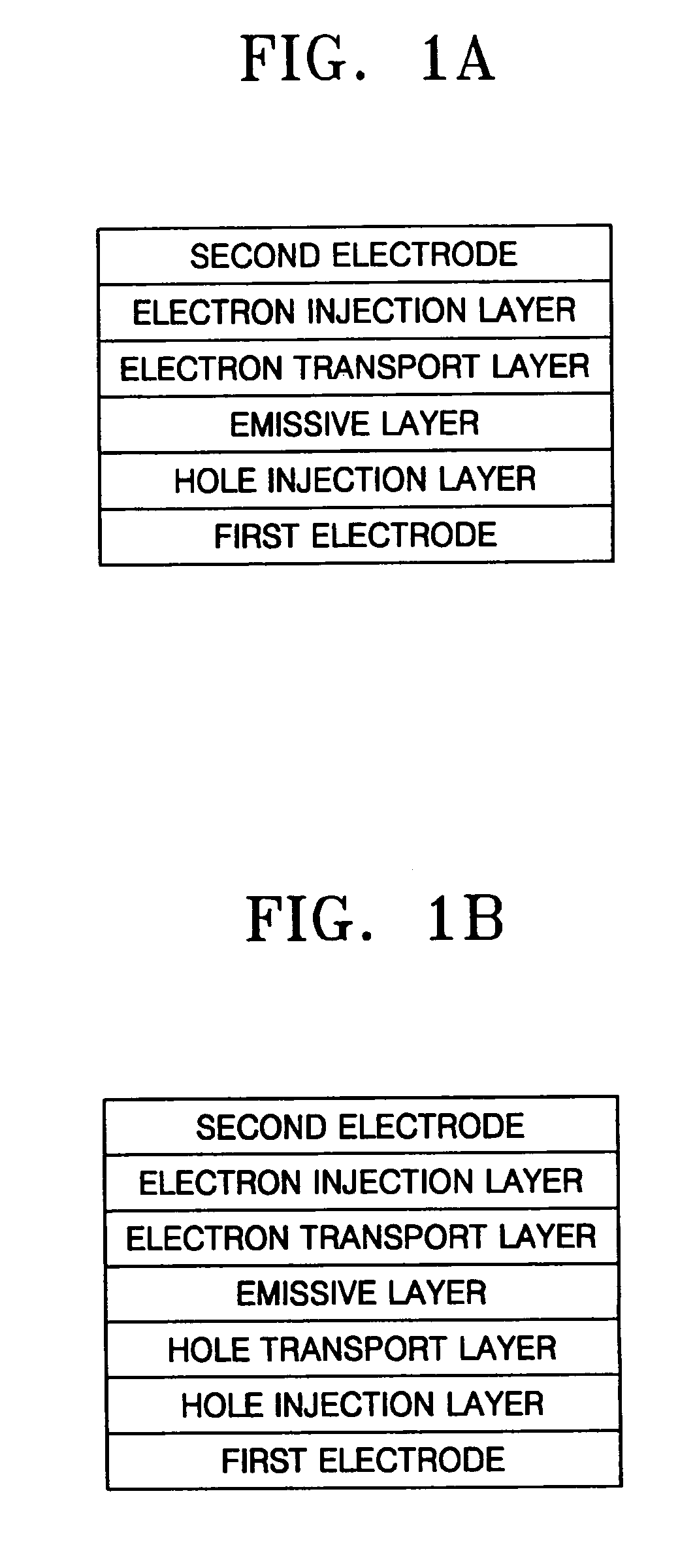
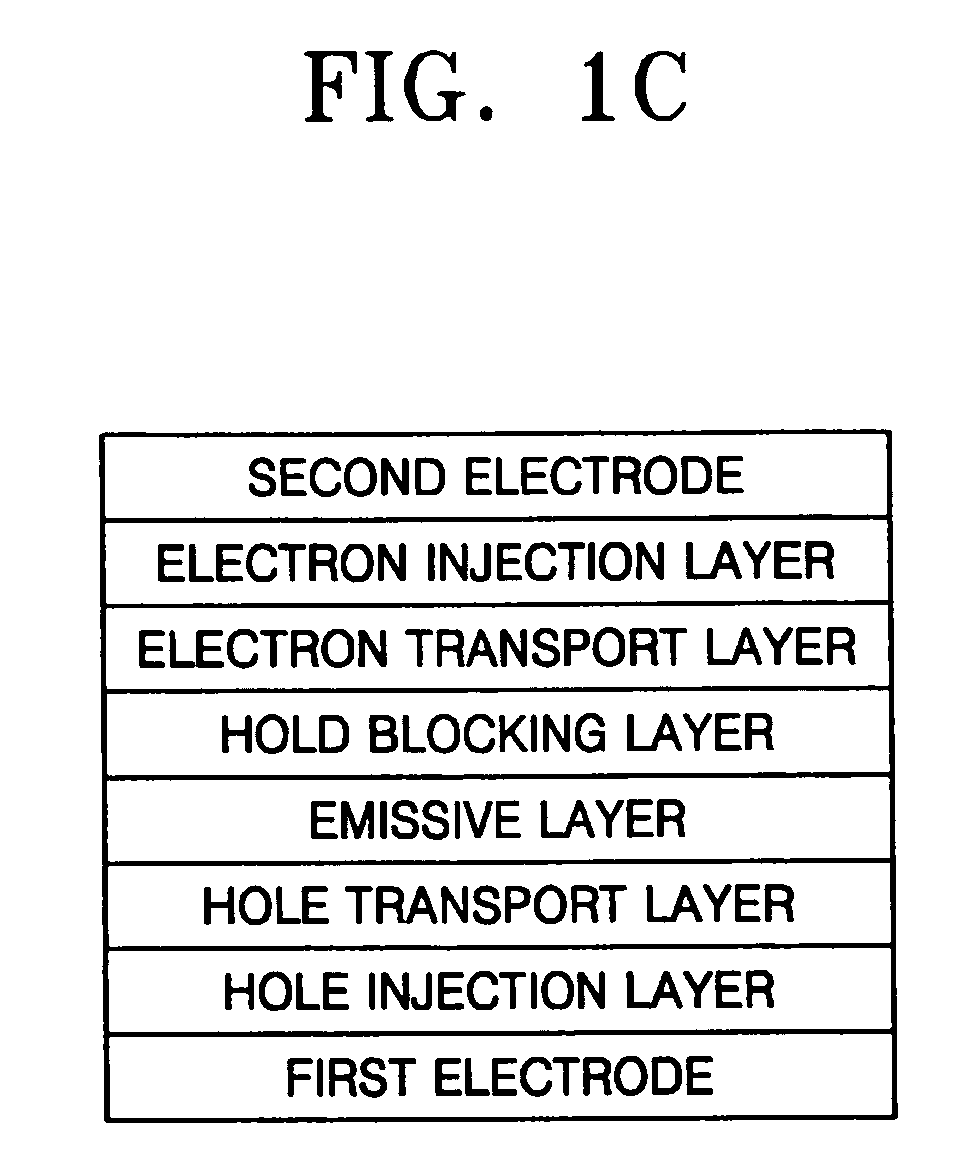
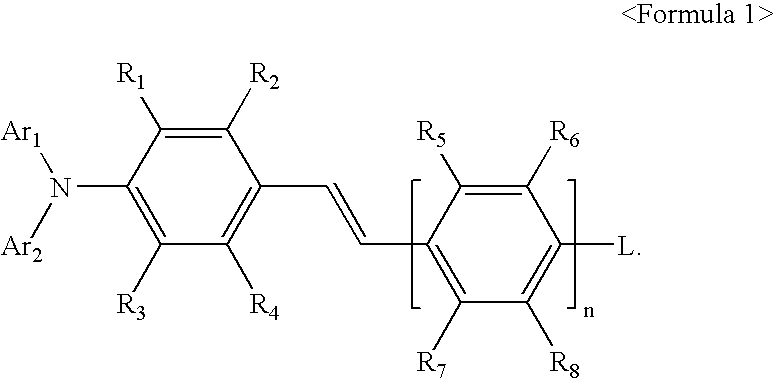
Aminostyryl compound, method of preparing the same, and organic light emitting device using the aminostyryl compound
a technology of aminostyryl compound and compound, which is applied in the field of aminostyryl compound, to achieve the effect of improving brightness, efficiency and color purity, and low driving voltag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 2
[0068] Compound 2 was synthesized through Reaction Scheme 2:
Synthesis of Intermediate B
[0069] 3 g (12 mmol) of 4-bromobenzyl bromide was mixed with 4.5 g (18 mmol) of P(OCH2CH3)3 and stirred at 185° C. for 6 hours. The result was cooled to room temperature to produce a crude product, which was purified using a silica gel column chromatography to produce 3.13 g (Yield 85%.) of Intermediate B
Synthesis of Intermediate C
[0070] Intermediate C (Yield 75%) was synthesized and purified in the same manner as in Comparative Example 1 except that Intermediate B was used instead of benzylphosphonic acid diethylester.
Synthesis of Compound 2
[0071] 1 g (2.35 mmol) of Intermediate C, 0.48 g (2.81 mmol) of 1-naphthaleneboronic acid, 0.135 g (0.12 mmol) of tetrakis (triphenylphosphine)paladium, 0.49 g (3.53 mmol) of K2CO3 were dissolved in 100 ml of toluene and 10 ml of water and stirred at a refluxing temperature for 48 hours. The reaction mixture was cooled to room temperature, and 100 ml of di
synthesis example 3
[0072] Compound 3 was synthesized through Reaction Scheme 3 below:
[0073] Compound 3 was produced in the same manner as in Synthesis Example 2, except that 4-biphenylyl boronic acid was used instead of 1-naphthalene boronic acid in the synthesis of Compound 2 of the Synthesis Example 2.
synthesis example 4
[0074] Compound 4 was synthesized through Reaction Scheme 4:
[0075] Compound 4 was produced in the same manner as in Synthesis Example 2, except that Intermediate D was used instead of 1-naphthlene boronic acid. Intermediate D was synthesized thorough Reaction Scheme 4′ below:
[0076] 5.35 ml (8.56 mmol) of 1.6M n-butyllithium was slowly dropped to 2 g (7.78 mmol) of 9-bromoanthracene dissolved in a solvent of 100 ml of tetrahydrofurane and reacted at −78° C. for one hour. 1.74 g (9.33 mmol) of 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane was added to the reaction mixture, stirred at room temperature for 18 hours, and 100 ml of methylenechloride was added thereto. The result was washed twice using 50 ml of water. Then, an organic layer was collected from the washed result and dried over anhydrous magnesiumsulfate to evaporate the solvent. As a result, a crude product was obtained. The crude product was purified using a silicagel column chromatography to produce 1.68 g (Yield 7
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap