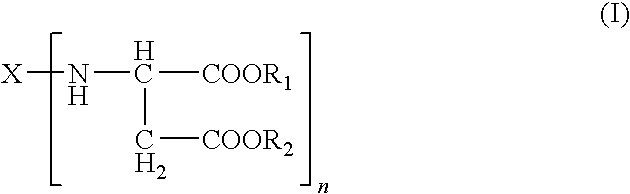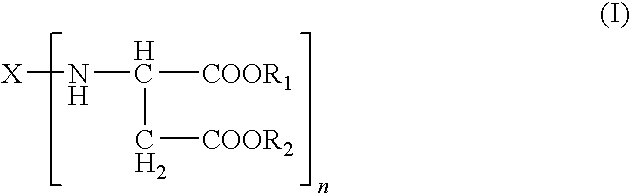Adhesive systems containing polyisocyanate prepolymers and aspartate-ester curing agents, processes for preparing the same, medical uses therefor and dispensing systems for the same
a technology of aspartate and curing agent, applied in the direction of adhesive types, polyurea/polyurethane adhesives, applications, etc., can solve the problems of cyanoacrylates only suitable for external surgical sutures, limited use, high cost, etc., and achieve the effect of simplifying application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Prepolymer A
[0090]465 g of HDI and 2.35 g of benzoyl chloride were placed in a 11 four-necked flask. 931.8 g of a polyether with an ethylene oxide content of 63% and a propylene oxide content of 37% (each based on the total alkylene oxide content) started with TMP (3-functional) were added within 2 hrs at 80° C. and then stirred for a further hour. Next, the excess HDI was distilled off by thin film distillation at 130° C. and 0.1 mm Hg. 980 g (71%) of the prepolymer with an NCO content of 2.53% were obtained. The residual monomer content was <0.03% HDI.
example 2
Aspartate B
[0091]1 mol of 2-methyl-1,5-diaminopentane was slowly added dropwise to 2 mols of diethyl maleate under a nitrogen atmosphere, so that the reaction temperature did not exceed 60° C. The mixture was then heated at 60° C. until diethyl maleate was no longer detectable in the reaction mixture, The product was purified by distillation.
example 2a
Aspartate Component Partially Pre-Extended with Isocyanate Group-Containing Prepolymer
[0092]1000 g of HDI (hexamethylene diisocyanate), 1 g of benzoyl chloride and 1 g of methyl para-toluenesulphonate were placed with stirring in a 41 four-necked flask. 1000 g of a bifunctional polypropylene glycol polyether with an average molecular weight of 2000 g / mol were added within 3 hours at 80° C. and then stirred for a further hour. The excess HDI was then distilled off by thin film distillation at 130° C. and 0.1 torr. The prepolymer obtained has an NCO content of 3.7%.
[0093]200 g of the prepolymer were fed with stirring at room temperature into 291 g of the aspartate B) from 2-methyl-1,5-diaminopentane in a 11 four-necked flask. This was stirred for a further two hours, until isocyanate groups were no longer detectable by IR spectroscopy. The product obtained had a viscosity of 3740 mPas and an NH equivalent weight of 460 g / eq.
Tissue Bonding Examples:
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Molar mass | aaaaa | aaaaa |
| Adhesivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap