Method of diagnosis of non-alcoholic fatty liver diseases
a non-alcoholic fatty liver and disease technology, applied in the direction of instruments, ict adaptation, material analysis, etc., can solve the problems of not being widely adopted, not being able to detect patients, and not being able to perform liver biopsy in such a large number of patients with metabolic risk factors, etc., to improve auroc, increase specificity and sensitivity, the effect of improving the sensitivity of the tes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
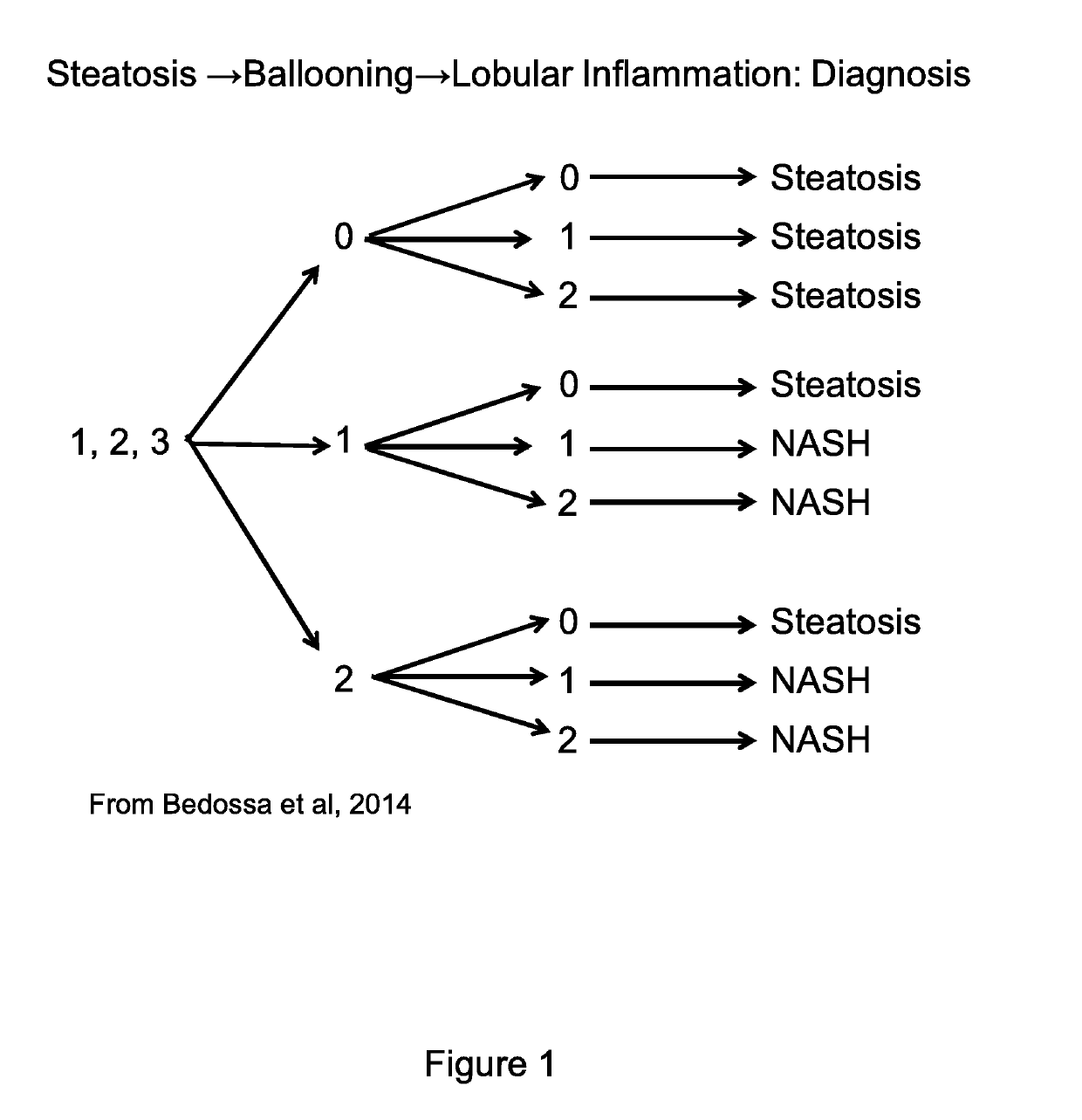
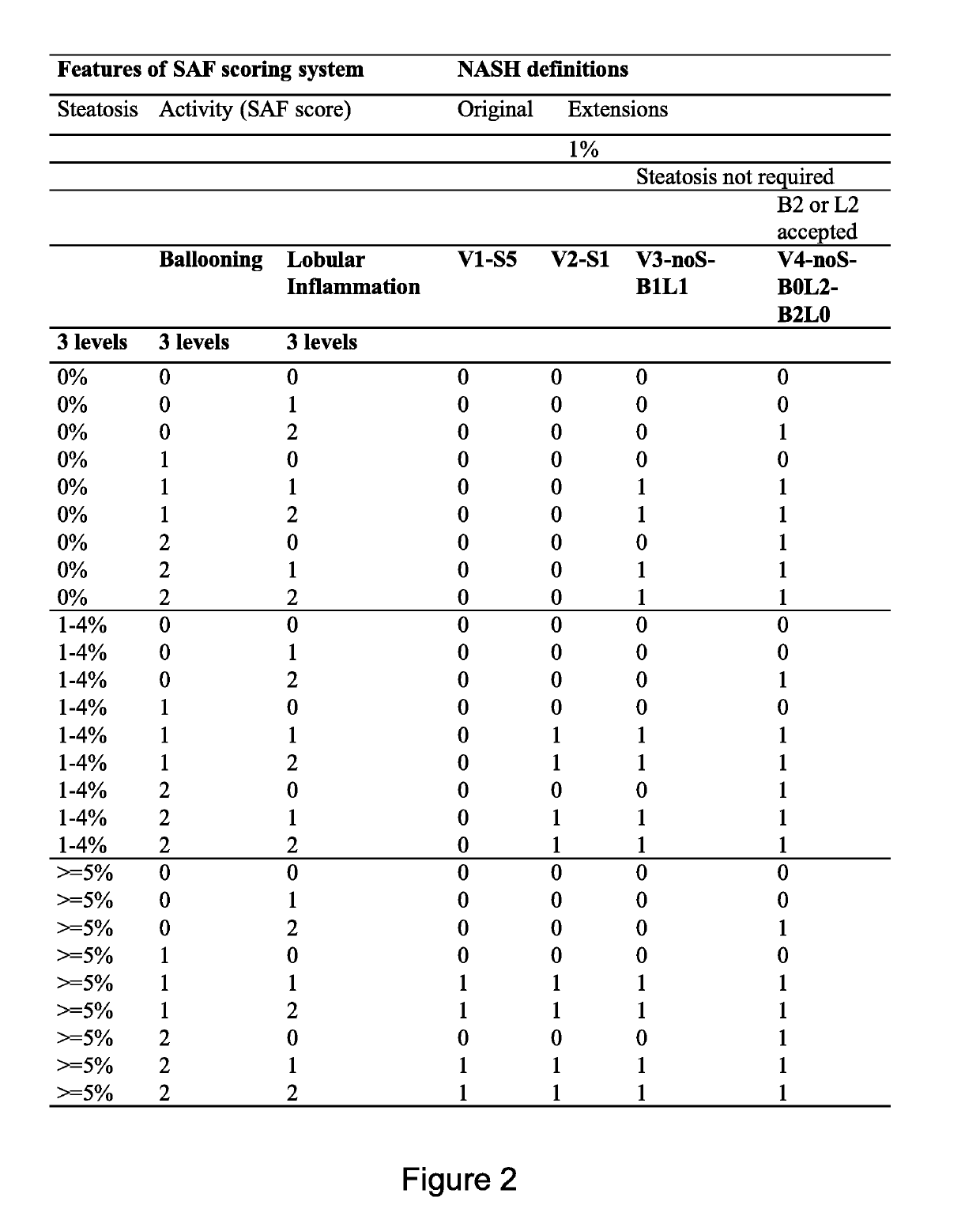
SAF Score and FLIP Algorithm
[0262]The classification was performed according to the following criteria, with the following paragraphs directly extracted from Bedossa et al (Hepatology, April 2014; August; 60(2):565-75; doi: 10.1002 / hep.27173).
[0263]For each biopsy a SAF score (Steatosis, Activity, Fibrosis) summarizing the main histological lesions was defined. This assesses both and separately the grade of steatosis (S), the grade of activity (A) and the stage of fibrosis (F), the latter according to NASH CRN (Kleiner et al Hepatology 2005; 41:1313-1321)
[0264]Steatosis was assessed by the percentage of hepatocytes containing large and medium-sized intracytoplasmic lipid droplets (but not foamy microvesicles), on a scale of 0 to 3 (S0: 67%).
[0265]Ballooning of hepatocytes was graded from 0 to 2 (0: normal hepatocytes with cuboidal shape, sharp angles and pink eosinophilic cytoplasm; 1: presence of clusters of hepatocytes with a rounded shape and pale cytoplasm, usually reticulated wh
example 2
gy for Developing the NASH-Non-Invasive Test
[0270]Recent simplified histological references were used for constructing new NITs, with modifications of these references due to the risk of sampling errors.
[0271]All histological scores were made according to the SAF classification as disclosed above.
[0272]For the binary histological diagnosis of NASH, it was decided to focus on ballooning and lobular inflammation, the specific features of necro-inflammatory histological activity as suggested by the histological FLIP algorithm. (Bedossa et al. Hepatology 2012, and Bedossa et al. Hepatology 2014).
[0273]It was indeed considered that the original FLIP algorithm has several limitations, the arbitrary choice of 5% of hepatocytes steatosis defining presence of steatosis, the exclusion of cases without steatosis for the diagnostic of NASH despite the severe activity grades, and the arbitrary choice of requiring both ballooning and lobular inflammation even if one of this feature reached a grade 2
example 3
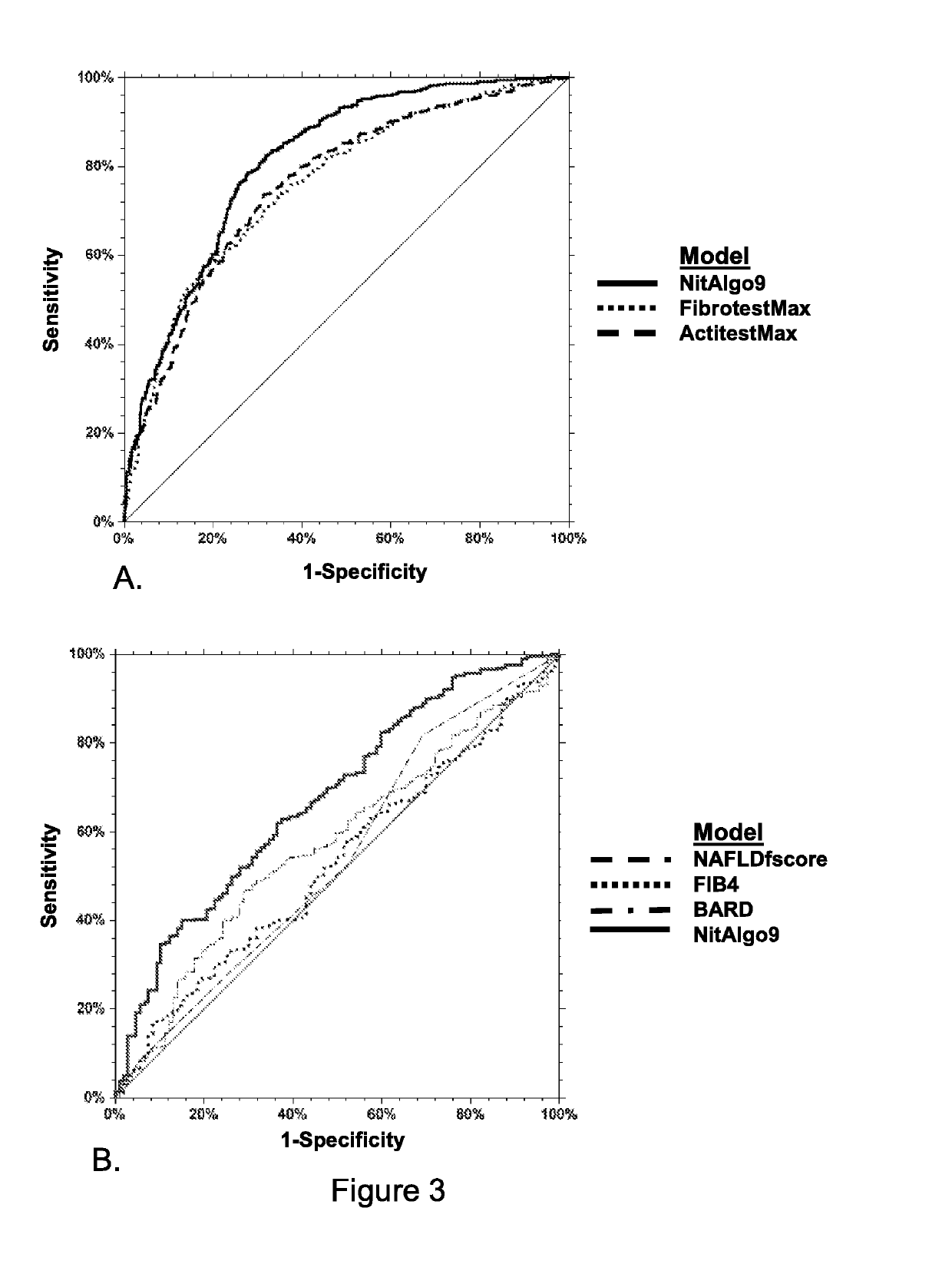
ion of the NIT-Algorithm for the Diagnosis of NASH Choice of the Markers to Use in these NITs
[0282]It was decided to use ALT, AST, that are known biomarkers of liver necrosis, as well as eight other liver components synthetized by the liver: apolipoprotein A1 (apoA1), haptoglobin, alpha-2-macroglobulin, total bilirubin, GGT, triglycerides, total cholesterol, fasting glucose and three patients characteristics (age, sex, and BMI).
[0283]Other markers could be equally used in the procedure herein described, such as markers that have been described in other liver NITs. One could cite γ-globulin, albumin, α1-globulin, α2-globulin, β-globulin, IL10, TGF-β1, apoA2, apoB, cytokeratin 18 and cytokeratin 19 components, platelets number, prothrombin level, hyaluronic acid, urea, N-terminal of type III pro-collagen, Tissue inhibitor metalloproteinase type-1 (TIMP-1), type IV collagen (Coll IV) and osteoprotegerin.
[0284]However, it was decided to use only the 13 elements as mentioned above, as the r
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap