Process for the preparation of microcapsules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Example
Example 1
Preparation of Capsules A to I
[0133]Microcapsules A to I were prepared with the following ingredients:
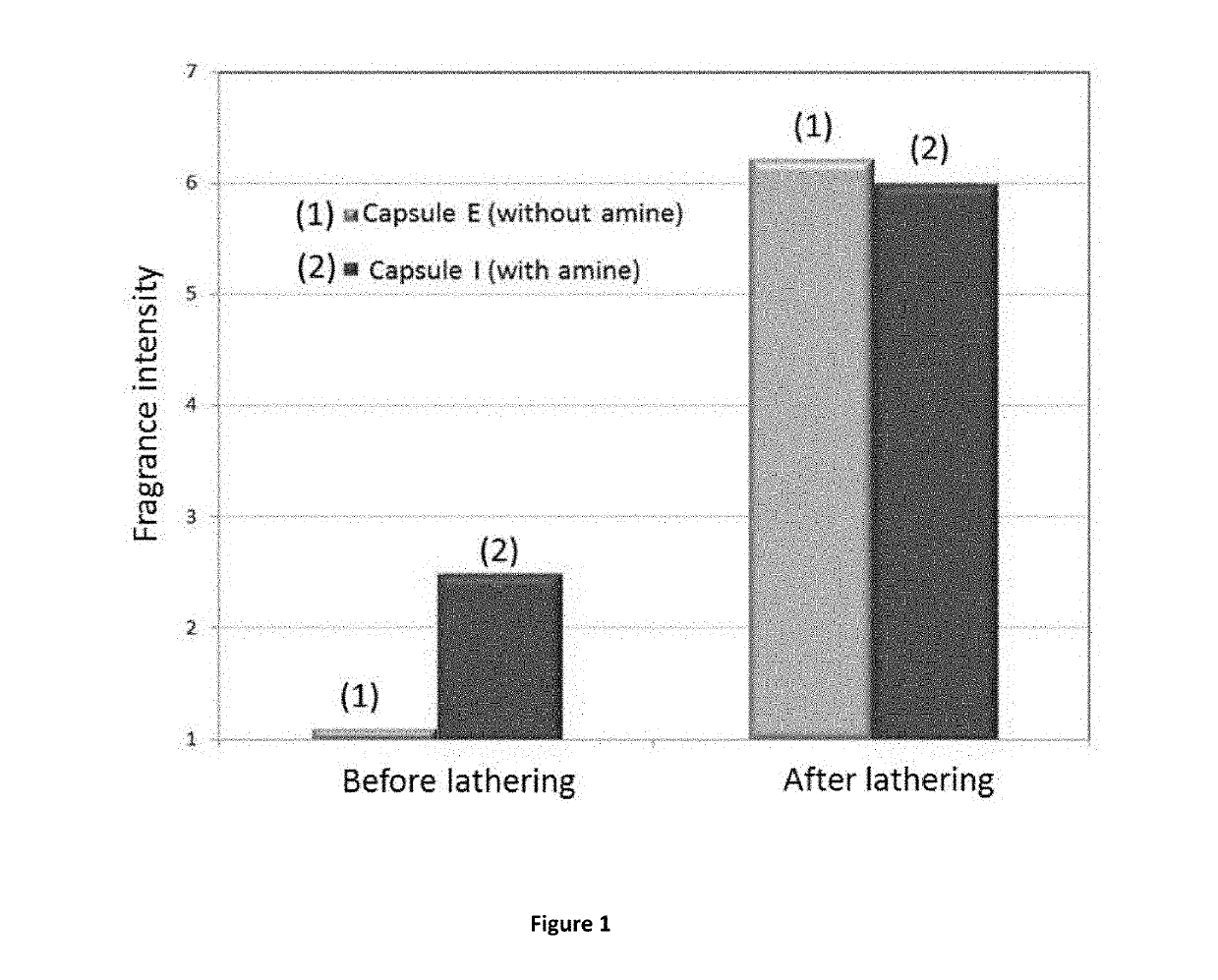
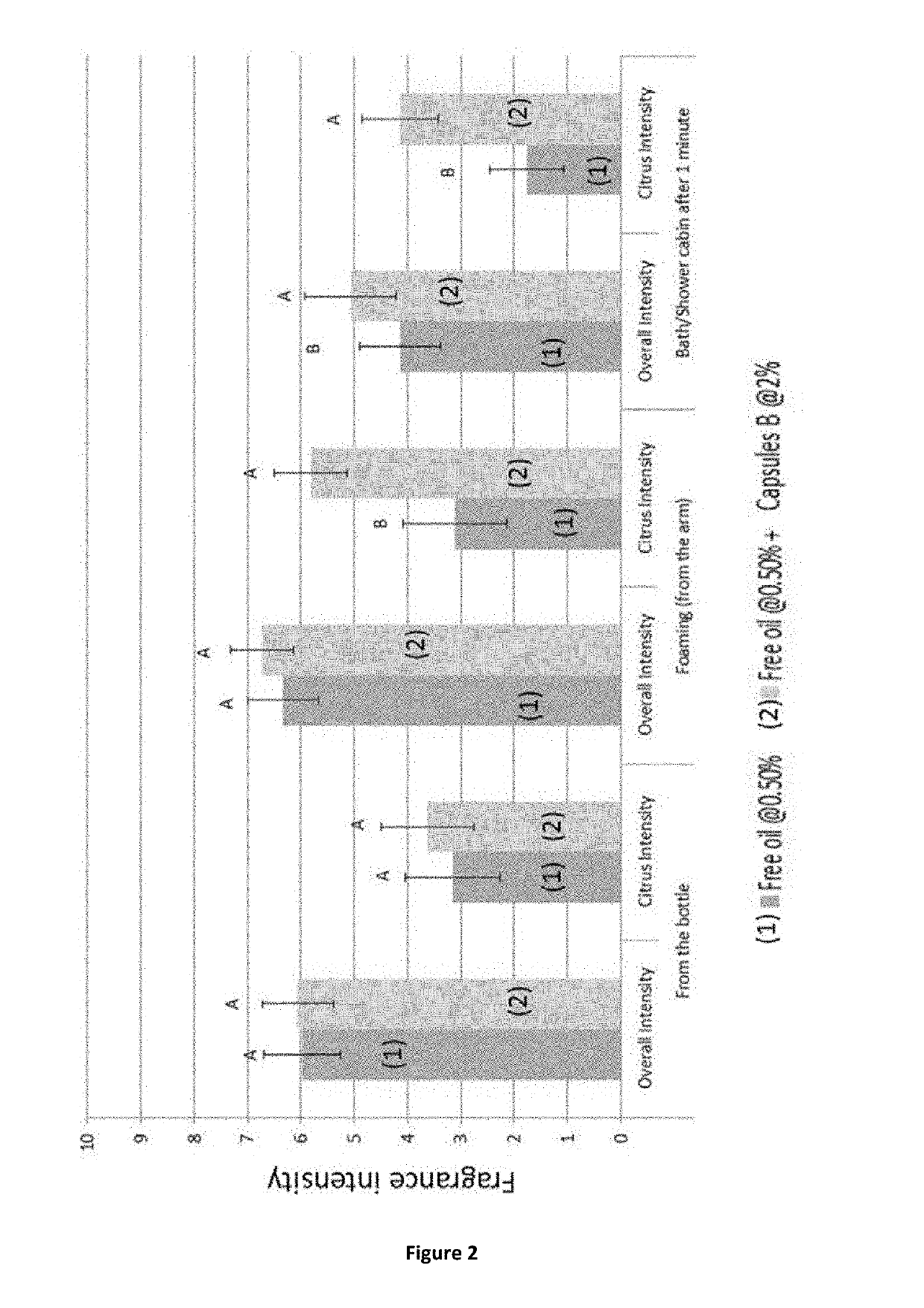
CapsulesCapsulesCapsulesCapsulesComparativeA to EFGHcapsule IAmountAmountAmountAmountAmountIngredient[g][g][g][g][g]Oil Phase21.727.033.738.620.9Perfume oil (perfume A) 1)variable24.330.434.819.65Polyisocyanate2)variable2.73.33.81.25Water phase77.7272.665.8560.9878.61Guanidine Carbonate3)00000.4Gum Arabic4)0.981.121.211.351.16Water76.7471.4864.6459.6377.051) see Table 22)Takenate ® D-110N (trimethylol propane adduct of xylylene diisocyanate); origin and trademark from Mitsui Chemicals, 75% polyisocyanate / 25% ethyl acetate3)origin: Fluka4)origin Alland & Robert
TABLE 2Composition of Perfume ARaw mat% in formulaSTIRRALLYL ACETATE4.5%BENZYL ACETATE0.9%ALDEHYDE C102.1%HEXYLCINNAMIC ALDEHYDE14.3% ALLYL CAPROATE0.7%Ethyl 2-methyl-pentanoate0.9%BENZYL BENZOATE35.3% CITRONELLYL NITRILE1.8%CORANOL 1)5.4%DIHYDROMYRCENOL5.4%FRUCTALATE ®2)5.4%HEDIONE ®3)14.9% LIMONENE2.4%LINALOOL1.0%METH
Example
Example 2
Preparation of Capsules J
[0139]The oil phase was prepared by admixing 1.6 g of polyisocyanate (trimethylol propane adduct of xylylene diisocyanate, Takenate® D-110N, origin and trademark from Mitsui Chemicals) with 25.4 g of perfume oil A (compositions defined in Table 2).
[0140]The aqueous phase was prepared by dissolving 0.1 g of sugar beet pectin (origin from CP Kelco) in 72.36 g of water. The emulsion was prepared by dispersing the perfume / polyisocyanate premix oil in the aqueous phase with the stirrer at 230 rpm. The temperature was raised to 70° C. and was kept at 70° C. for 1 h30 to allow the curing of the capsules. At this point, capsules were formed, cross-linked and stable. The mixture was left to cool down to room temperature.
[0141]After encapsulation and use of the Takenate D-110N to produce the capsule wall, the residual level of unreacted polyisocyanate in the perfume oil was very low and therefore the internal core of the capsule was essentially made of the per
Example
Example 3
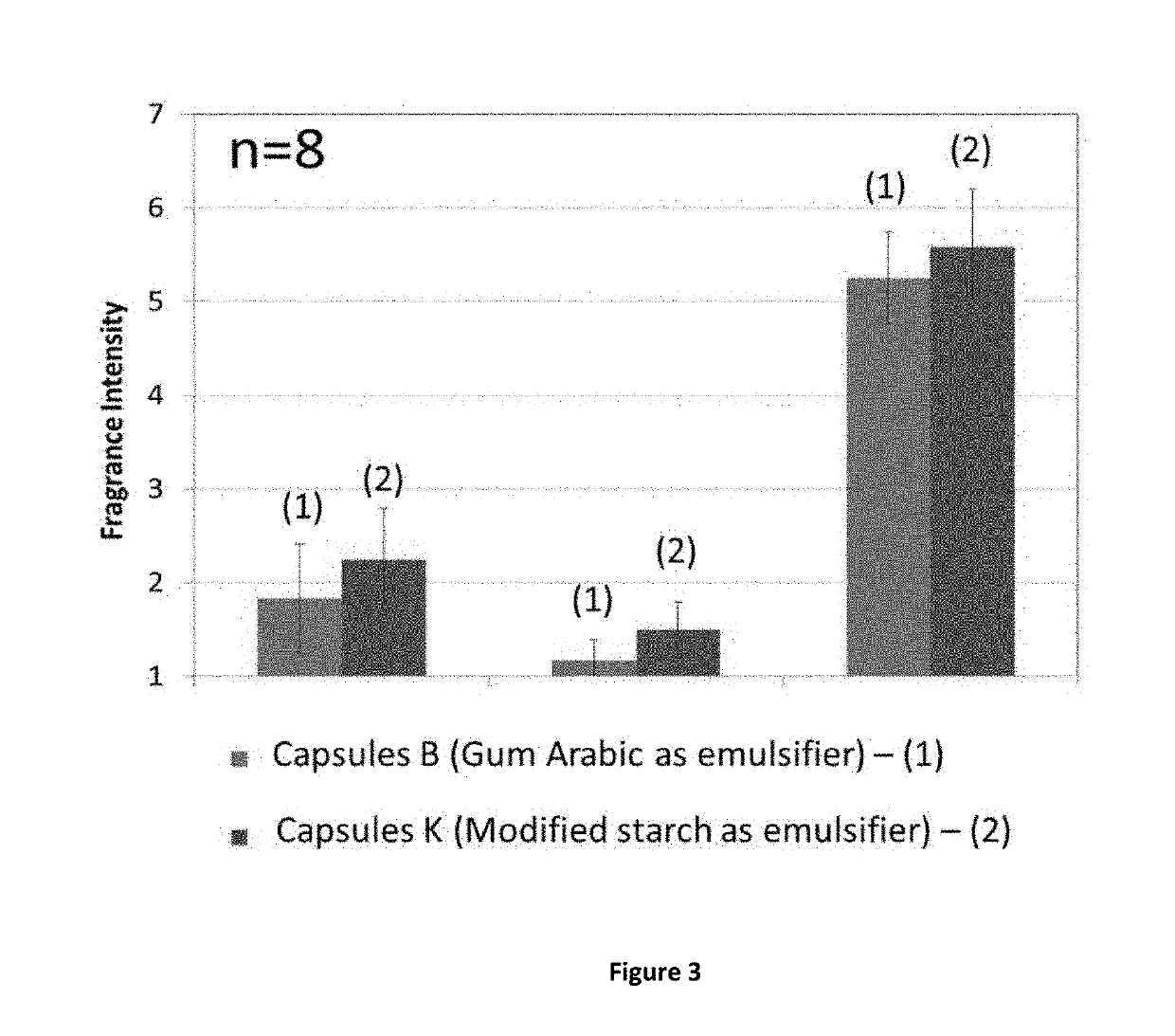
Preparation of Capsules K
[0143]The oil phase was prepared by admixing 2.2 g of polyisocyanate (trimethylol propane adduct of xylylene diisocyanate, Takenate® D-110N, origin and trademark from Mitsui Chemicals) with 19.6 g of perfume oil A (compositions defined in Table 2).
[0144]The aqueous phase was prepared by dissolving 1.2 g of modified starch (Hicap-100® origin from Ingredion Inc) in 76.7 g of water. The emulsion was prepared by dispersing the perfume / polyisocyanate premix oil in the aqueous phase with the stirrer at 230 rpm. The temperature was raised to 70° C. and was kept at 70° C. for 1 h30 to allow the curing of the capsules. At this point, capsules were formed, cross-linked and stable. The mixture was left to cool down to room temperature.
[0145]After encapsulation and use of the Takenate D-110N to produce the capsule wall, the residual level of unreacted polyisocyanate in the perfume oil was very low and therefore the internal core of the capsule was essentially ma
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap