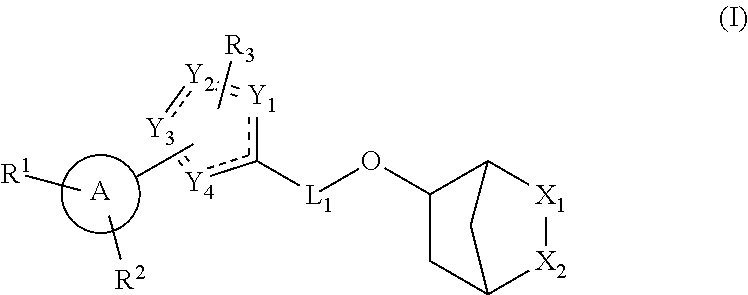
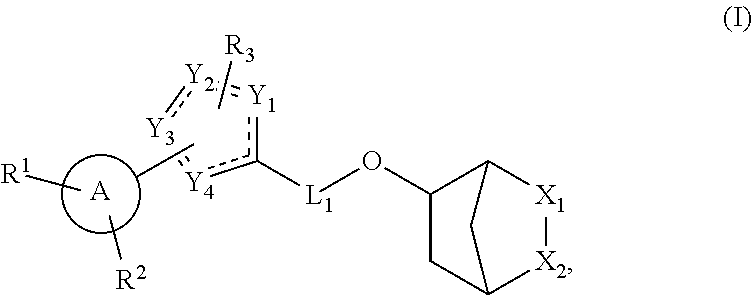
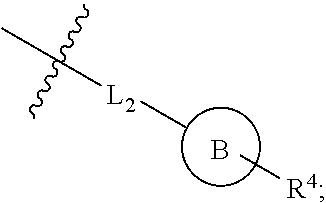
Hormone receptor modulators for treating metabolic mutagenic and fibrotic conditions and disorders
a hormone receptor and modulator technology, applied in the field of nuclear hormone receptor modulators, farnesoid x receptor (fxr), can solve the problems of toxicity of fxr activators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ate. Benzyl (1S,4S,5R)-5-hydroxy-2-azabicyclo[2.2.1]heptane-2-carboxylate (C-1) and (1S,4R,6S)-Benzyl 6-hydroxy-2-aza-bicyclo[2.2.1]heptane-2-carboxylate (C-2)
[0369]
[0370]Step 1. Benzyl (1S,4R)-2-azabicyclo[2.2.1]hept-5-ene-2-carboxylate (C-1b)
[0371]To a 250-mL 3-necked round-bottom flask purged and maintained under an inert atmosphere of nitrogen was added a solution of LiAlH4 (2.15 g, 56.65 mmol, 1.25 equiv.) in tetrahydrofuran (80 mL). A solution of (1S,4R)-2-azabicyclo[2.2.1]hept-5-en-3-one C-1a (5 g, 45.82 mmol, 1.0 equiv.) in tetrahydrofuran (45 mL) was added dropwise with stirring at 0° C. The mixture was stirred at 23° C. for 3 h, and then continued at 60° C. for 24 h. After cooling to room temperature, water (5 mL) was added. The resulting mixture was diluted with 250 mL of tetrahydrofuran, and the solids were removed by filtration. The filtrate was cooled to 0° C. and TEA was added (9.1 g, 89.93 mmol, 2.0 equiv.) dropwise followed by the dropwise addition of benzyl chloroform
example 2
ate. Benzyl (1R,4R,5S)-5-hydroxy-2-azabicyclo[2.2.1]heptane-2-carboxylate (C-4) and Benzyl (1R,4S,6R)-6-hydroxy-2-azabicyclo[2.2.1]heptane-2-carboxylate (C-5)
[0376]
[0377]Step 1. Benzyl (1S,4S,5S)-5-[(4-nitrophenyl)carbonyloxy]-2-azabicyclo[2.2.1]heptane-2-carboxylate (C-3a)
[0378]To a 250-mL round-bottom flask was added a solution of benzyl (1S,4S,5R)-5-hydroxy-2-azabicyclo[2.2.1]heptane-2-carboxylate C-1 (1.03 g, 4.17 mmol, 1.0 equiv.) in tetrahydrofuran (50 mL) and 4-nitrobenzoic acid (1.05 g, 6.28 mmol, 1.50 equiv.). The reaction mixture was cooled to 0° C. and PPh3 was added (1.64 g, 6.25 mmol, 1.50 equiv) in several batches followed by dropwise addition of DIAD (1.26 g, 6.23 mmol, 1.50 equiv). The resulting mixture was stirred at room temperature overnight and then concentrated under reduced pressure. The residue was purified by silica gel column chromatography eluting with ethyl acetate / petroleum ether (25:75) to give 1.6 g (97%) of benzyl (1S,4S,5S)-5-[(4-nitrophenyl)carbonylox
example 3
ates. Benzyl (1R,4R,5S)-5-hydroxy-2-azabicyclo[2.2.1]heptane-2-carboxylate (C-4) and Benzyl (1R,4S,6R)-6-hydroxy-2-azabicyclo[2.2.1]heptane-2-carboxylate (C-5)
[0381]
[0382]Step 1. Benzyl (1R,4S)-2-azabicyclo[2.2.1]hept-5-ene-2-carboxylate (C-2)
[0383]A solution of (1R,4S)-2-azabicyclo[2.2.1]hept-5-en-3-one C-4a (5.0 g, 45.8 mmol) in anhydrous THF (50 mL) was added slowly to a solution of LAH (28.7 mL, 57.3 mmol, 2M solution in THF) in anhydrous THF (50 mL) under a nitrogen atmosphere at 0° C. The resulting mixture was then stirred at room temperature for 3 h and then heated at 60° C. for 24 h. The mixture was cooled to 0° C. and H2O (5.0 mL) was added carefully to the mixture. The resulting white suspension was filtered through a Celite pad and the pad was washed with anhydrous THF (250.0 mL). The clear filtrate was cooled to 0° C. and then treated with trimethylamine (12.8 mL, 91.6 mmol) and CbzCl (10.3 mL, 68.7 mmol). The reaction mixture was slowly warmed to room temperature and stirr
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap