Preparation method of cyclosporin A haptin and enzymelinked immunosorbent quantitative detection kit of cyclosporin A
An enzyme-linked immunosorbent assay and cyclosporine technology, applied in measuring devices, instruments, analytical materials, etc., can solve the problem of limited economic affordability of organ transplant patients, the large gap in the level of medical and health testing institutions, and improve the standards of hospitals and testing centers. Entry threshold and other issues to achieve the effect of shortening detection time, high sensitivity, and low pre-processing requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
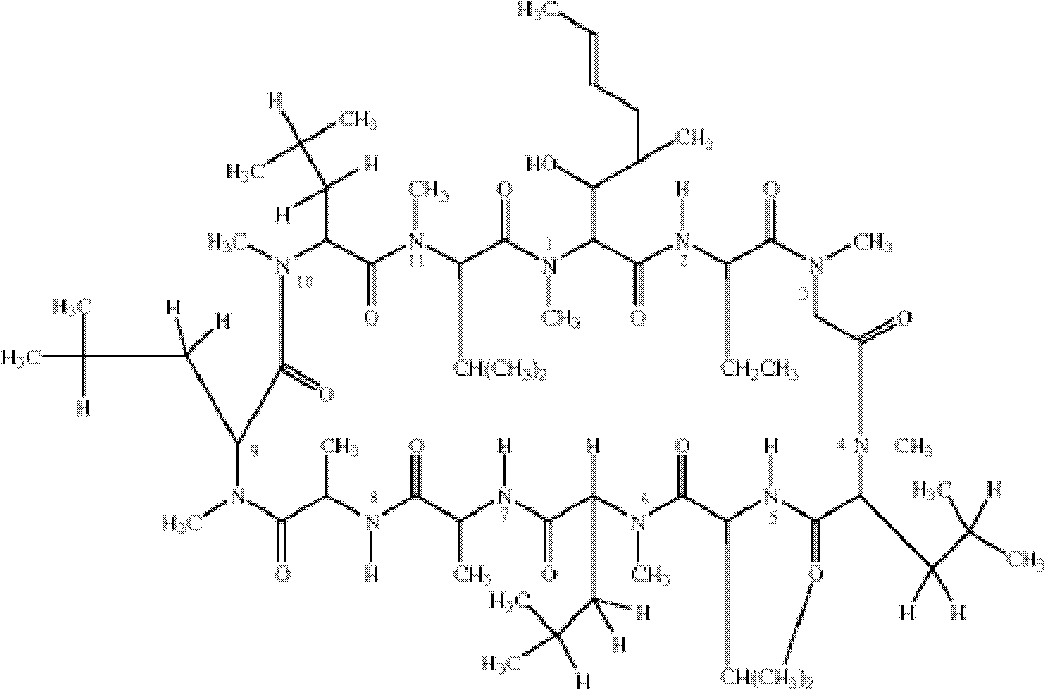
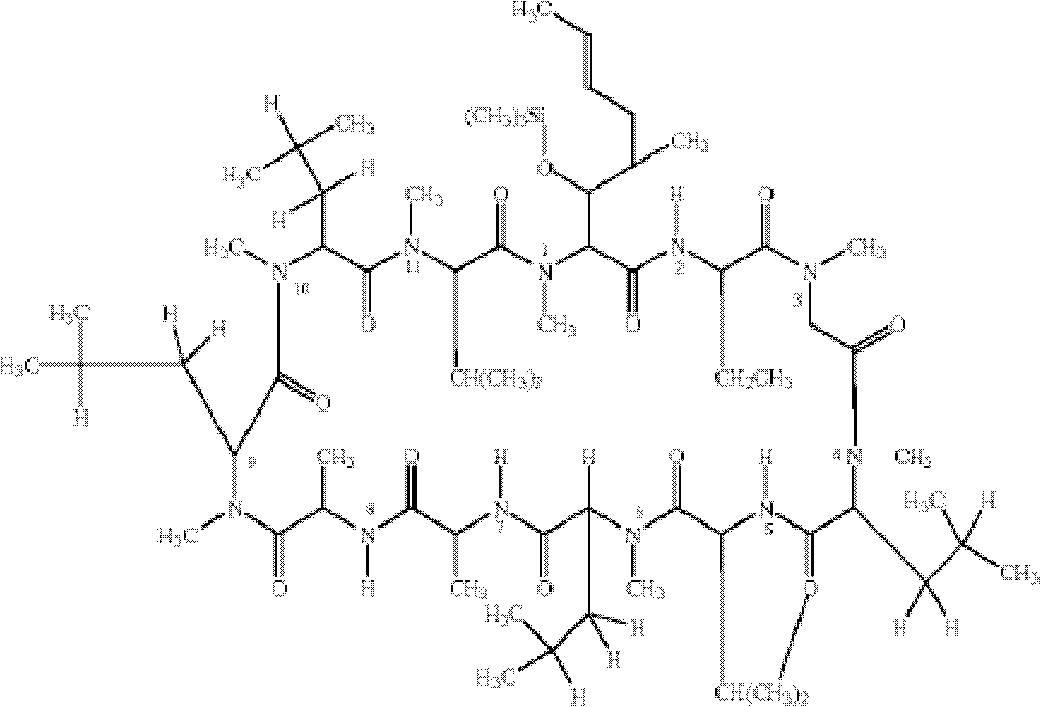
[0036] Example 1. Preparation of cyclosporine A hapten
[0037] 1) Weigh 1800mg of cyclosporine A (CsA) and dissolve it in 6ml of pyridine, then add 6ml of dichloromethane dropwise, and stir overnight at room temperature in an argon-filled environment;
[0038]2) The solid residue after vacuum evaporation and drying of the above product was dissolved in dichloromethane, separated and purified by gel column to obtain TMS-CsA (1700 mg, 89%);
[0039] 3) Add the above product (1000mg, 0.78mmol) into 0.2ml of diethyl ether, add 30ml of toluene, add sodium hydride (450mg, form a 50% mineral oil suspension, extract with toluene), and stir at room temperature for 30min under the condition of argon. After cooling to 4°C, add 6ml of ethylene oxide with a syringe, cover the reactor, seal with paraffin, stir at room temperature for 24 hours, add 100ml of water, acidify with (1N) HCL, and add 200ml of dichloromethane. After separating the organic phase, add 100ml of water to elute, dry and
Embodiment 2
[0044] Example 2, Preparation and use of a kit in which cyclosporine A monoclonal antibody is used as the coating source and the marker is an enzyme-labeled hapten
[0045] 1. The detection principle of the kit using cyclosporine A monoclonal antibody as the coating source and the enzyme-labeled hapten as the marker is as follows:
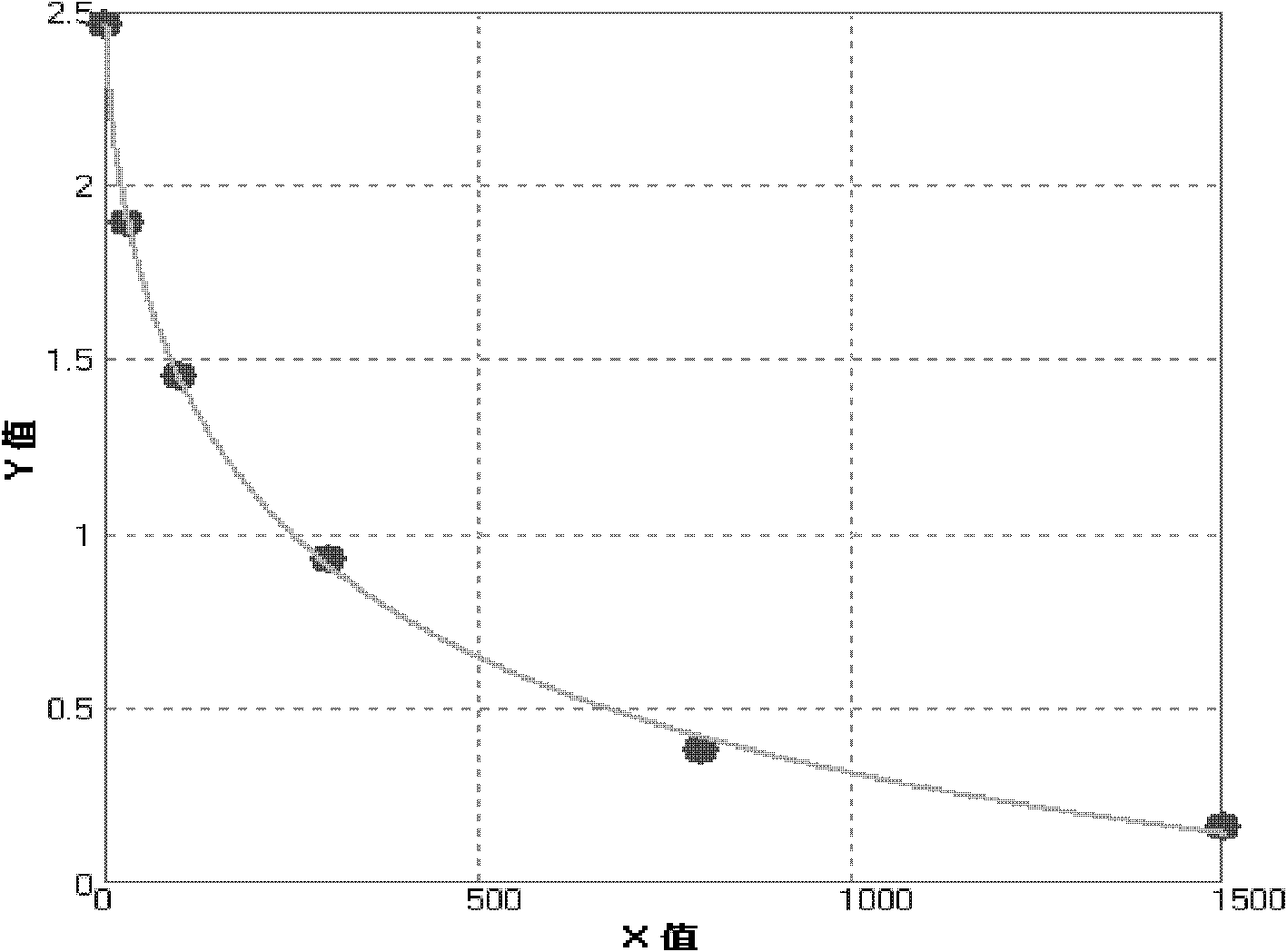
[0046] Cyclosporine A antibody is pre-coated on the microwell strip, and the cyclosporine A in the sample competes with the enzyme-labeled cyclosporine A and the pre-coated anti-cyclosporine A antibody on the microwell strip In this way, the absorbance value of the sample is negatively correlated with the content of cyclosporine A contained in the sample, and the amount of cyclosporine A in the sample can be obtained by comparing with the standard curve.
[0047] 2. The composition of the kit with cyclosporine A monoclonal antibody as the coating source and the enzyme-labeled hapten as the marker can generally include the following:
[0048] 1. ELISA
Embodiment 3
[0104] Embodiment 3, the kit that is used to detect the drug concentration of cyclosporine A in human whole blood can also have following several kinds:
[0105] 1. The original coating is a conjugate of cyclosporin A hapten and carrier protein, and the enzyme-labeled secondary antibody is an enzyme-labeled kit
[0106] 1. The working principle of this kit is:
[0107] When the coating on the microwell strip of the microtiter plate is originally a conjugate of cyclosporine A hapten and carrier protein, after adding the standard solution or sample solution to the microwell of the microtiter plate, add anti-cyclosporine Cyclosporin A in the sample competes with the cyclosporine A-coupled antigen on the microtiter plate, and then the enzyme-labeled anti-antibody is added for amplification, and the substrate chromogenic solution is used to develop the antibody. In this way, the absorbance value of the sample is negatively correlated with the content of cyclosporine A, and the conten
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap