Application of oxygen carrier with perovskite structure in chemical looping hydrogen production and preparation thereof
A perovskite structure and oxygen carrier technology, which is applied in the field of chemical chain hydrogen production, can solve the problems of low dispersion of metal oxides, low cycle reactivity, and limited oxygen loading rate, so as to promote the oxidation-reduction process, Accelerated cycle efficiency and low cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
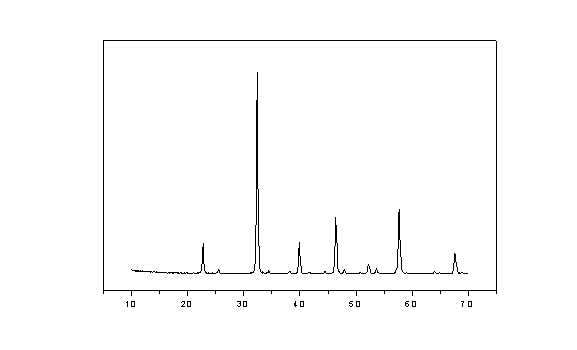
Image
Examples
Embodiment 1
[0018] Take 20.7gCo(NO 3 ) 2 ·6H 2 O and 1.9gCu(NO 3 ) 2 ·3H 2 Put O into a 500mL beaker so that the molar ratio of Co to Cu is 0.9 / 0.1, add 100mL of distilled water, then place the beaker in a water bath at 80°C, stir at 400rpm, and stir until completely dissolved. Take 34.3g La(NO 3 ) 3 ·6H 2 O, put it in a beaker with 100mL distilled water, stir until it is completely dissolved. Then add the lanthanum nitrate solution dropwise to the mixed solution of cobalt nitrate and copper nitrate, and stir while adding dropwise. Take 40g of citric acid, the molar ratio of citric acid to the total amount of metal ions is 1.2:1, put it into a 100mL beaker and stir until it is completely dissolved. At this time, after the above mixed solution is stirred for 30 minutes, slowly add the citric acid solution, while Add dropwise while stirring. After stirring for 5 hours, the brown solution had been dehydrated and turned into a viscous gel. The gel was taken out and placed in a drying
Embodiment 2
[0020] Take 16.1gCo(NO 3 ) 2 ·6H 2 O and 5.7gCu(NO 3 ) 2 ·3H 2 Put O into a 500mL beaker, make the molar ratio of Co to Cu 0.7 / 0.3, and stir until completely dissolved. Add 100mL of distilled water, then place the beaker in a water bath at 80°C with a stirring speed of 400rpm. Take 34.3g La(NO 3 ) 3 ·6H 2 O, put it in a beaker with 100mL distilled water, stir until it is completely dissolved. Then add the lanthanum nitrate solution dropwise to the cobalt nitrate and copper nitrate solutions, and stir while adding. Take 67g of citric acid, the molar ratio of citric acid to metal ions is 1.2:1, put it into a 100mL beaker and stir until it is completely dissolved. After the above mixed solution is stirred for 30 minutes, slowly add the citric acid solution, while adding While stirring. After stirring for 5 hours, the brown solution had been dehydrated and turned into a viscous gel. The gel was taken out and placed in a drying oven at 110° C. to dry overnight. Then take
Embodiment 3
[0022] Take 11.5g Co(NO 3 ) 2 ·6H 2 O, 9.5gCu(NO 3 ) 2 ·3H 2Put O into a 500mL beaker, make the molar ratio of Co to Cu 0.5 / 0.5, add 100mL of distilled water, then place the beaker in a water bath at 80°C, stir at 400rpm, and stir until completely dissolved. Take 34.3g La(NO 3 ) 3 ·6H 2 O, put it in a beaker with 100mL distilled water, stir until it is completely dissolved. Then add the lanthanum nitrate solution dropwise to the mixed solution of cobalt nitrate and copper nitrate, and stir while adding dropwise. Take 40g of citric acid, the molar ratio of citric acid to the total amount of metal ions is 1.2:1, put it into a 100mL beaker and stir until it is completely dissolved. After the above mixed solution is stirred for 30 minutes, slowly add the citric acid solution, while adding While stirring. After stirring for 5 hours, the brown solution had been dehydrated and turned into a viscous gel. The gel was taken out and placed in a drying oven at 110° C. to dry over
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap