Clinical payment network system and methods
A clinically tested, defined technology, applied in the field of financial management systems, that can address issues such as increased complexity of learning data, regulatory constraints, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
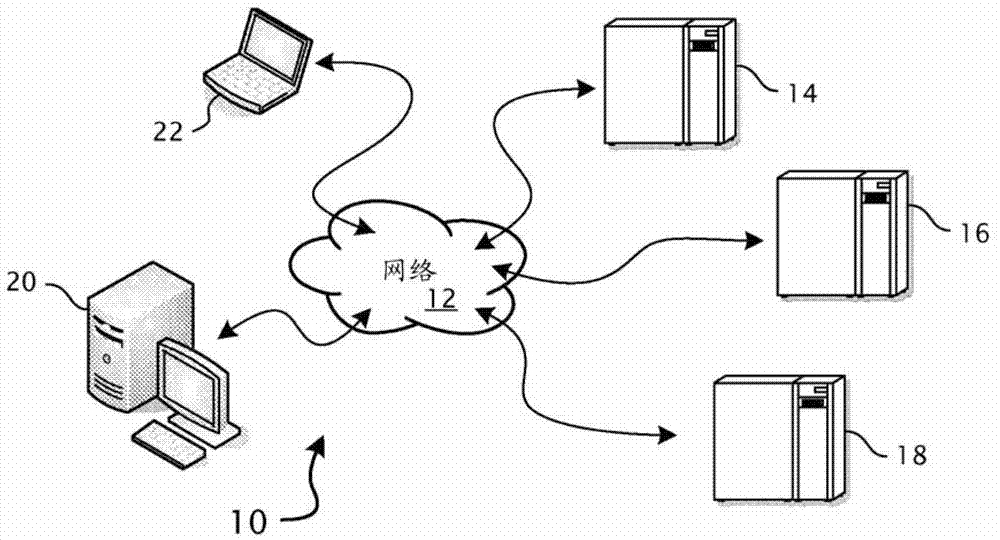
[0033] The invention is described in the preferred context of a distributed computer system capable of supporting global access and use in the context of managing clinical trials, more specifically with regard to the processing of financial transactions. However, the present invention is able to properly handle all operational transactions within clinical trials, and further, operational transactions within distributed computer systems that require complex and typically deterministic evaluations of operational conditions. These capabilities will become apparent from the ensuing detailed description of the invention, wherein like reference numerals are used to refer to like parts in one or more of the drawings.
[0034] refer to figure 1 , the preferred environment for distributed data processing system 10 typically includes heterogeneous computer platforms interconnected by network 12, which is traditionally the global Internet. Servers 14, 16, and 18 are typically located in a
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap