Probucol controlled-release tablet for treatment of hypercholesteremia and production method thereof
A technology of hypercholesterolemia and probucol, which is applied in the fields of pharmaceutical formulations, metabolic diseases, active ingredients of sulfur/selenium/tellurium, etc., can solve the problem of low bioavailability of probucol tablets and the absence of probucol preparations Reports on sustained-release tablets and other issues, to achieve drug safety, reduce side effects, and reduce the number of times of taking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
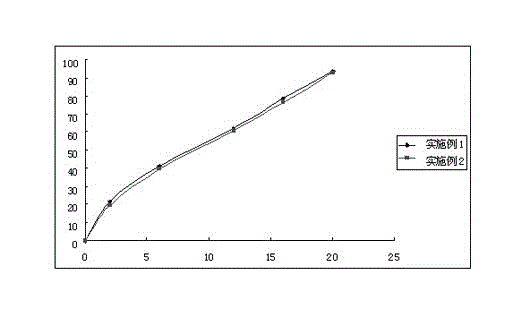
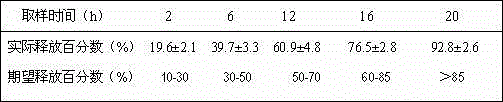
Embodiment 1
[0037] Example 1 Probucol Sustained Release Tablets (500mg)
[0038] Prescription composition:
[0039] Element mg / tablet Probuka 500 lactose 80 HPMCK15M 220 PVP-K30 30 5% starch slurry Appropriate amount Magnesium stearate 8 Gastric Opadry 20
[0040] production method:
[0041] Pass the prescribed amount of Probucol through a 100-mesh sieve, and pass the prescribed amount of HPMC-K15M, lactose and PVP-K30 through a 80-mesh sieve. Mix the above raw and auxiliary materials evenly, make soft material with 5% starch slurry, granulate with 18-mesh sieve, dry at 50°C, granulate with 20-mesh sieve, add prescription amount of magnesium stearate, mix evenly, and compress into tablets to obtain Chips, spare;
[0042] Put the above tablet cores into the coating pan, the speed is 30 rpm, the temperature is controlled at 50-60°C, and the prepared gastric-soluble Opadry film coating solution (the prescribed amount of gastric-solub
Embodiment 2
[0043] Example 2 Probucol Sustained Release Tablets (1000mg)
[0044] Prescription composition:
[0045] Element mg / tablet Probuka 1000 microcrystalline cellulose 180 HPMCK100M 350 PVP-K30 50 5% starch slurry Appropriate amount Magnesium stearate 15 Gastric Opadry 35
[0046] production method:
[0047] Pass the prescribed amount of Probucol through a 100-mesh sieve, and pass the prescribed amount of HPMC-K100M, microcrystalline cellulose and PVP-K30 through an 80-mesh sieve respectively. Mix the above raw and auxiliary materials evenly, make soft material with 5% starch slurry, granulate with 18-mesh sieve, dry at 50°C, granulate with 20-mesh sieve, add prescription amount of magnesium stearate, mix evenly, and compress into tablets to obtain Chips, spare;
[0048] Put the above tablet cores into the coating pan, the speed is 30 rpm, the temperature is controlled at 50-60°C, and the prepared gastric-soluble Opadry
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap