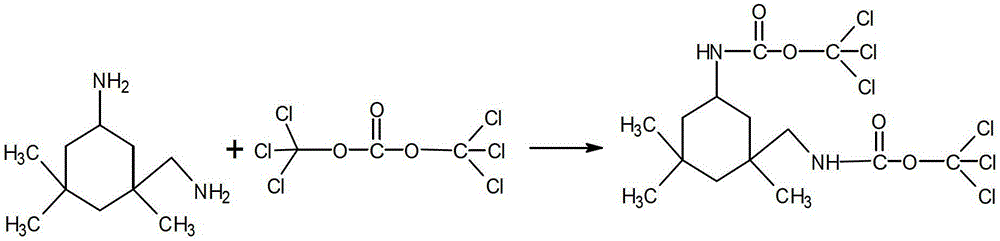
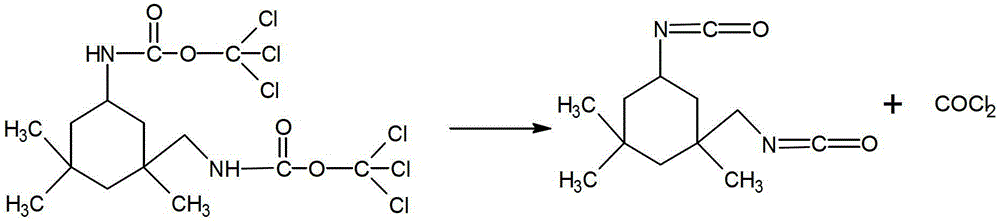
Synthetic method of isophorone diisocyanate
A technology of isophorone diisocyanate and isophorone diamine is applied in the field of synthesis of isophorone diisocyanate, which can solve the problems of danger in use, transportation and storage, high volatility and difficulty in metering, and achieve product Post-processing is simple and easy, high purity, and safe to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0022] Example 1
[0023] (1) Dissolve 17 g of isophorone diamine in 195.5 g of chloroform, and dissolve 29.7 g of solid phosgene in 342 g of chloroform;
[0024] (2) Add the isophorone diamine solution dropwise to the solid phosgene solution at -20°C, and react for 7 hours. During this process, nitrogen is continuously introduced for gas replacement;
[0025] (3) The above reaction solution was heated to reflux, reacted for 5 hours, after the reaction was completed, cooled, filtered with suction, and the filtrate was distilled under reduced pressure to obtain 20.7 g of product. The product yield was 93.2% and the purity was 97.5%.
Example Embodiment
[0026] Example 2
[0027] (1) Dissolve 34 g of isophorone diamine and 0.64 g of N,N-dimethylformamide in 193 g of chloroform, and dissolve 29.7 g of solid phosgene in 168.3 g of chloroform;
[0028] (2) Add the isophorone diamine solution dropwise to the solid phosgene solution at 5°C, and react for 2 hours. During this process, nitrogen is continuously introduced for gas replacement;
[0029] (3) The above reaction liquid was heated to reflux, reacted for 2 hours, after the reaction was completed, cooled, filtered with suction, and the filtrate was distilled under reduced pressure to obtain the product 19.0, the product yield was 85.5%, and the purity was 94.3%.
Example Embodiment
[0030] Example 3
[0031] (1) Dissolve 25.5 g of isophorone diamine and 2.76 g of paraformaldehyde in 203.5 g of dichloroethane, and dissolve 29.7 g of solid phosgene in 240.3 g of dichloroethane;
[0032] (2) Add the isophorone diamine solution dropwise to the solid phosgene solution at -10°C, and react for 4.5 hours. During this process, nitrogen is continuously introduced for gas replacement;
[0033] (3) The above reaction solution was heated to reflux, reacted for 8 hours, after the reaction was completed, cooled, filtered with suction, and the filtrate was distilled under reduced pressure to obtain 20.2 g of product. The product yield was 90.8% and the purity was 93.1%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap